Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis
- PMID: 15967814
- PMCID: PMC2171636
- DOI: 10.1083/jcb.200411155
Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis
Abstract
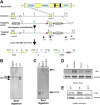
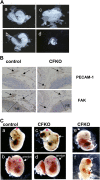
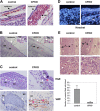
Focal adhesion kinase (FAK) is a critical mediator of signal transduction by integrins and growth factor receptors in a variety of cells including endothelial cells (ECs). Here, we describe EC-specific knockout of FAK using a Cre-loxP approach. In contrast to the total FAK knockout, deletion of FAK specifically in ECs did not affect early embryonic development including normal vasculogenesis. However, in late embryogenesis, FAK deletion in the ECs led to defective angiogenesis in the embryos, yolk sac, and placenta, impaired vasculature and associated hemorrhage, edema, and developmental delay, and late embryonic lethal phenotype. Histologically, ECs and blood vessels in the mutant embryos present a disorganized, detached, and apoptotic appearance. Consistent with these phenotypes, deletion of FAK in ECs isolated from the floxed FAK mice led to reduced tubulogenesis, cell survival, proliferation, and migration in vitro. Together, these results strongly suggest a role of FAK in angiogenesis and vascular development due to its essential function in the regulation of multiple EC activities.
Figures





References
-
- Cary, L.A., J.F. Chang, and J.L. Guan. 1996. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J. Cell Sci. 109:1787–1794. - PubMed
-
- Cattelino, A., S. Liebner, R. Gallini, A. Zanetti, G. Balconi, A. Corsi, P. Bianco, H. Wolburg, R. Moore, B. Oreda, et al. 2003. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J. Cell Biol. 162:1111–1122. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

