An alternative interpretation of the amyloid Abeta hypothesis with regard to the pathogenesis of Alzheimer's disease
- PMID: 15967987
- PMCID: PMC1166615
- DOI: 10.1073/pnas.0503181102
An alternative interpretation of the amyloid Abeta hypothesis with regard to the pathogenesis of Alzheimer's disease
Abstract
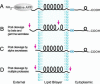
Alzheimer's disease is a complex neurodegenerative process that is believed to be due to the accumulation of short, hydrophobic peptides derived from amyloid precursor proteins by proteolytic cleavage. It is widely believed that these Abeta peptides are secreted into the extracellular spaces of the CNS, where they assemble into toxic oligomers that kill neurons and eventually form deposits of senile plaques. This essay explores the possibility that a fraction of these Abeta peptides never leave the membrane lipid bilayer after they are generated, but instead exert their toxic effects by competing with and compromising the functions of intramembranous segments of membrane-bound proteins that serve many critical functions. Based on the presence of shared amino acid sequences containing GxxG motifs, I speculate that accumulations of intramembranous Abeta peptides might affect the functions of amyloid precursor protein itself and the assembly of the PS1, Aph1, Pen 2, Nicastrin complex.
Figures

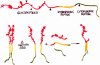

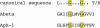
References
-
- Wilquet, V. & De Strooper, B. (2004) Neurobiology 14, 582-588. - PubMed
-
- Selcoe, D. J. (2001) Neuron 32, 177-180. - PubMed
-
- Oddo, S., Caccamo, A., Shepherd, J. D, Murphy, M. P., Golde, T. E., Kayed, R., Metherate, R., Mattson, M. P., Akbari, Y. & Laferla, F. M. (2003) Neuron 39, 409-421. - PubMed
-
- D'Andrea, M. R., Nagele, R. G., Wang, H.-Y., Peterson, P. A. & Lee, D. H. S. (2001) Histopathology 38, 120-134. - PubMed
-
- Kienlen-Campard, P., Miolet, S., Tasiaux, B. & Octave, J.-N. (2002) J. Biol. Chem. 277, 15666-15670. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

