Cell intrinsic alterations underlie hematopoietic stem cell aging
- PMID: 15967997
- PMCID: PMC1153718
- DOI: 10.1073/pnas.0503280102
Cell intrinsic alterations underlie hematopoietic stem cell aging
Abstract
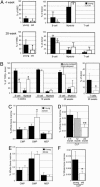
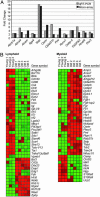
Loss of immune function and an increased incidence of myeloid leukemia are two of the most clinically significant consequences of aging of the hematopoietic system. To better understand the mechanisms underlying hematopoietic aging, we evaluated the cell intrinsic functional and molecular properties of highly purified long-term hematopoietic stem cells (LT-HSCs) from young and old mice. We found that LT-HSC aging was accompanied by cell autonomous changes, including increased stem cell self-renewal, differential capacity to generate committed myeloid and lymphoid progenitors, and diminished lymphoid potential. Expression profiling revealed that LT-HSC aging was accompanied by the systemic down-regulation of genes mediating lymphoid specification and function and up-regulation of genes involved in specifying myeloid fate and function. Moreover, LT-HSCs from old mice expressed elevated levels of many genes involved in leukemic transformation. These data support a model in which age-dependent alterations in gene expression at the stem cell level presage downstream developmental potential and thereby contribute to age-dependent immune decline, and perhaps also to the increased incidence of leukemia in the elderly.
Figures

References
-
- Schlessinger, D. & Van Zant, G. (2001) Mech. Ageing Dev. 122, 1537-1553. - PubMed
-
- Morrison, S. J., Wandycz, A. M., Akashi, K., Globerson, A. & Weissman, I. L. (1996) Nat. Med. 2, 1011-1016. - PubMed
-
- Ikuta, K., Kina, T., MacNeil, I., Uchida, N., Peault, B., Chien, Y. H. & Weissman, I. L. (1990) Cell 62, 863-874. - PubMed
-
- Hardy, R. R., Wei, C. J. & Hayakawa, K. (2004) Immunol. Rev. 197, 60-74. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

