Aggregometry detects platelet hyperreactivity in healthy individuals
- PMID: 15972447
- PMCID: PMC1634759
- DOI: 10.1182/blood-2005-03-1290
Aggregometry detects platelet hyperreactivity in healthy individuals
Abstract
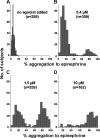
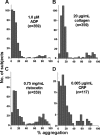
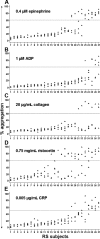
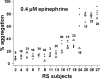
Aggregometry is widely used to assess platelet function, but its use in identifying platelet hyperreactivity is poorly defined. We studied platelet aggregation in 359 healthy individuals using the agonists adenosine diphosphate (ADP), epinephrine, collagen, collagen-related peptide, and ristocetin. We also assessed the reproducibility of these assays in 27 subjects by studying them repeatedly on at least 4 separate occasions. Healthy subjects exhibited considerable interindividual variability in aggregation response to agonists, especially at concentrations lower than those typically used in clinical laboratories. For each agonist tested at these submaximal concentrations, a small proportion of individuals demonstrated an unusually robust aggregation response. Subjects who exhibited such in vitro hyperreactivity to one agonist tended to demonstrate a similar response to others, suggesting that hyperreactivity is a global characteristic of platelets. Epinephrine and collagen-related peptide were especially reliable and efficient in detecting hyperreactivity. For epinephrine, excellent reproducibility persisted for up to 3 years, and hyperreactivity was associated with female sex and higher fibrinogen levels (P < .02). We recommend these assays as appropriate candidates for future studies requiring accurate assessment of increased platelet reactivity. These include clinical studies to improve risk assessment for arterial thrombosis, as well as genetic studies to establish determinants of the hyperreactive platelet phenotype.
Figures

References
-
- Refaai MA, Laposata M. Platelet aggregation. In: Michelson AD, ed. Platelets. San Diego, CA: Academic Press; 2002: 291-296.
-
- Coller BS. Platelet aggregation by ADP, collagen, and ristocetin: a critical review of methodology and analysis. In: Schmidt RM, ed. CRC Handbook Series in Clinical Laboratory Science. Section I. Boca Raton, FL: CRC Press; 1979: 381-396.
-
- Sixma JJ. Methods for platelet aggregation. In: Mannucci PM, Gorini S, eds. Platelet Function and Thrombosis. Vol 34. New York, NY: Plenum Press; 1972: 79-96. - PubMed
-
- Tiffany ML. Technical considerations for platelet aggregation and related problems. Crit Rev Clin Lab Sci. 1983;19: 27-69. - PubMed
-
- George JN, Caen JP, Nurden AT. Glanzmann's thrombasthenia: the spectrum of clinical disease. Blood. 1990;75: 1383-1395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

