Arrangement of the translocator of the autotransporter adhesin involved in diffuse adherence on the bacterial surface
- PMID: 15972470
- PMCID: PMC1168569
- DOI: 10.1128/IAI.73.7.3851-3859.2005
Arrangement of the translocator of the autotransporter adhesin involved in diffuse adherence on the bacterial surface
Abstract
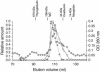
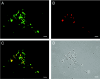
Autotransporters of gram-negative bacteria are single-peptide secretion systems that consist of a functional N-terminal alpha-domain ("passenger") fused to a C-terminal beta-domain ("translocator"). How passenger proteins are translocated through the outer membrane has not been resolved, and at present essentially three different models are discussed. In the widely accepted "hairpin model" the passenger proteins are translocated through a channel formed by the beta-barrel of the translocator that is integrated in the outer membrane. This model has been challenged by a recent proposal for a general autotransporter model suggesting that there is a hexameric translocation pore that is generated by the oligomerization of six beta-domains. A third model suggests that conserved Omp85 participates in autotransporter integration and passenger protein translocation. To examine these models, in this study we investigated the presence of putative oligomeric structures of the translocator of the autotransporter adhesin involved in diffuse adherence (AIDA) in vivo by cross-linking techniques. Furthermore, the capacity of isolated AIDA fusion proteins to form oligomers was studied in vitro by several complementary analytical techniques, such as analytical gel filtration, electron microscopy, immunogold labeling, and cross-linking of recombinant autotransporter proteins in which different passenger proteins were fused to the AIDA translocator. Our results show that the AIDA translocator is mostly present as a monomer. Only a fraction of the AIDA autotransporter was found to form dimers on the bacterial surface and in solution. Higher-order structures, such as hexamers, were not detected either in vivo or in vitro and can therefore be excluded as functional moieties for the AIDA autotransporter.
Figures




Similar articles
-
Barriers to folding of the transmembrane domain of the Escherichia coli autotransporter adhesin involved in diffuse adherence.Biochemistry. 2005 Mar 22;44(11):4533-45. doi: 10.1021/bi0475121. Biochemistry. 2005. PMID: 15766284
-
Mutations affecting the biogenesis of the AIDA-I autotransporter.Res Microbiol. 2007 May;158(4):348-54. doi: 10.1016/j.resmic.2007.02.006. Epub 2007 Mar 6. Res Microbiol. 2007. PMID: 17446047
-
Characterization of the essential transport function of the AIDA-I autotransporter and evidence supporting structural predictions.J Bacteriol. 1999 Nov;181(22):7014-20. doi: 10.1128/JB.181.22.7014-7020.1999. J Bacteriol. 1999. PMID: 10559167 Free PMC article.
-
Self-associating autotransporters, SAATs: functional and structural similarities.Int J Med Microbiol. 2006 Aug;296(4-5):187-95. doi: 10.1016/j.ijmm.2005.10.002. Int J Med Microbiol. 2006. PMID: 16600681 Review.
-
Structures and functions of autotransporter proteins in microbial pathogens.Int J Med Microbiol. 2011 Aug;301(6):461-8. doi: 10.1016/j.ijmm.2011.03.003. Epub 2011 May 25. Int J Med Microbiol. 2011. PMID: 21616712 Review.
Cited by
-
Proteolytic processing is not essential for multiple functions of the Escherichia coli autotransporter adhesin involved in diffuse adherence (AIDA-I).J Bacteriol. 2006 Dec;188(24):8504-12. doi: 10.1128/JB.00864-06. Epub 2006 Oct 13. J Bacteriol. 2006. PMID: 17041044 Free PMC article.
-
Type V Secretion: the Autotransporter and Two-Partner Secretion Pathways.EcoSal Plus. 2010 Sep;4(1):10.1128/ecosalplus.4.3.6. doi: 10.1128/ecosalplus.4.3.6. EcoSal Plus. 2010. PMID: 26443787 Free PMC article.
-
Surface display of proteins by gram-negative bacterial autotransporters.Microb Cell Fact. 2006 Jun 20;5:22. doi: 10.1186/1475-2859-5-22. Microb Cell Fact. 2006. PMID: 16787545 Free PMC article.
-
Comparative analysis of the biochemical and functional properties of C-terminal domains of autotransporters.J Bacteriol. 2010 Nov;192(21):5588-602. doi: 10.1128/JB.00432-10. Epub 2010 Aug 27. J Bacteriol. 2010. PMID: 20802036 Free PMC article.
-
The periplasmic folding of a cysteineless autotransporter passenger domain interferes with its outer membrane translocation.J Bacteriol. 2006 Jun;188(11):4111-6. doi: 10.1128/JB.01949-05. J Bacteriol. 2006. PMID: 16707702 Free PMC article.
References
-
- Benz, I., and M. A. Schmidt. 1992. AIDA-I, the adhesin involved in diffuse adherence of the diarrhoeagenic Escherichia coli strain 2787 (O126:H27), is synthesized via a precursor molecule. Mol. Microbiol. 6:1539-1546. - PubMed
-
- Benz, I., and M. A. Schmidt. 2001. Glycosylation with heptose residues mediated by the aah gene product is essential for adherence of the AIDA-I adhesin. Mol. Microbiol. 40:1403-1413. - PubMed
-
- Benz, I., and M. A. Schmidt. 2003. Never say never again: protein glycosylation in pathogenic bacteria. Mol. Microbiol. 45:267-276. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

