Tyrosine phosphorylation of the chlamydial effector protein Tarp is species specific and not required for recruitment of actin
- PMID: 15972471
- PMCID: PMC1168552
- DOI: 10.1128/IAI.73.7.3860-3868.2005
Tyrosine phosphorylation of the chlamydial effector protein Tarp is species specific and not required for recruitment of actin
Abstract
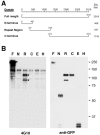
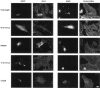
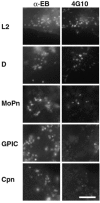
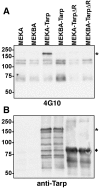
Chlamydiae are obligate intracellular pathogens that efficiently induce their endocytosis by susceptible eukaryotic host cells. Recently, a Chlamydia trachomatis type III secreted effector protein, Tarp, was found to be translocated and tyrosine phosphorylated at the site of entry and associated with the recruitment of actin that coincides with endocytosis. C. trachomatis Tarp possesses up to six direct repeats of approximately 50 amino acids each. The majority of the tyrosine residues are found within this repeat region. Here we have ectopically expressed distinct domains of Tarp in HeLa 229 cells and demonstrated that tyrosine phosphorylation occurs primarily within the repeat region, while recruitment of actin is mediated by the C-terminal domain of the protein. A comparison of other sequenced chlamydial genomes revealed that each contains an ortholog of Tarp, although Chlamydia muridarum, Chlamydophila caviae, and Chlamydophila pneumoniae Tarp lack the large repeat region. Immunofluorescence and immunoblotting using an antiphosphotyrosine antibody show no evidence of phosphotyrosine at the site of entry of C. muridarum, C. caviae, and C. pneumoniae, although each species similarly recruits actin. Ectopic expression of full-length C. trachomatis and C. caviae Tarp confirmed that both recruit actin but only C. trachomatis Tarp is tyrosine phosphorylated. The data indicate that the C-terminal domain of Tarp is essential for actin recruitment and that tyrosine phosphorylation may not be an absolute requirement for actin recruitment. The results further suggest the potential for additional, unknown signal transduction pathways associated specifically with C. trachomatis.
Figures



Similar articles
-
A chlamydial type III translocated protein is tyrosine-phosphorylated at the site of entry and associated with recruitment of actin.Proc Natl Acad Sci U S A. 2004 Jul 6;101(27):10166-71. doi: 10.1073/pnas.0402829101. Epub 2004 Jun 15. Proc Natl Acad Sci U S A. 2004. PMID: 15199184 Free PMC article.
-
Chlamydia trachomatis Tarp harbors distinct G and F actin binding domains that bundle actin filaments.J Bacteriol. 2013 Feb;195(4):708-16. doi: 10.1128/JB.01768-12. Epub 2012 Nov 30. J Bacteriol. 2013. PMID: 23204471 Free PMC article.
-
Targeted Disruption of Chlamydia trachomatis Invasion by in Trans Expression of Dominant Negative Tarp Effectors.Front Cell Infect Microbiol. 2016 Aug 23;6:84. doi: 10.3389/fcimb.2016.00084. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27602332 Free PMC article.
-
Pathogenic Puppetry: Manipulation of the Host Actin Cytoskeleton by Chlamydia trachomatis.Int J Mol Sci. 2019 Dec 21;21(1):90. doi: 10.3390/ijms21010090. Int J Mol Sci. 2019. PMID: 31877733 Free PMC article. Review.
-
[Effector proteins of Clamidia].Mol Biol (Mosk). 2009 Nov-Dec;43(6):963-83. Mol Biol (Mosk). 2009. PMID: 20088373 Review. Russian.
Cited by
-
Chlamydia trachomatis species-specific induction of ezrin tyrosine phosphorylation functions in pathogen entry.Infect Immun. 2007 Dec;75(12):5669-77. doi: 10.1128/IAI.01096-07. Epub 2007 Oct 1. Infect Immun. 2007. PMID: 17908813 Free PMC article.
-
Phylogenetic analysis of Chlamydia trachomatis Tarp and correlation with clinical phenotype.Infect Immun. 2010 Sep;78(9):3678-88. doi: 10.1128/IAI.00515-10. Epub 2010 Jul 6. Infect Immun. 2010. PMID: 20605986 Free PMC article.
-
Chlamydial intracellular survival strategies.Cold Spring Harb Perspect Med. 2013 May 1;3(5):a010256. doi: 10.1101/cshperspect.a010256. Cold Spring Harb Perspect Med. 2013. PMID: 23637308 Free PMC article. Review.
-
Comprehensive in silico prediction and analysis of chlamydial outer membrane proteins reflects evolution and life style of the Chlamydiae.BMC Genomics. 2009 Dec 29;10:634. doi: 10.1186/1471-2164-10-634. BMC Genomics. 2009. PMID: 20040079 Free PMC article.
-
Chlamydia trachomatis secretion of proteases for manipulating host signaling pathways.Front Microbiol. 2011 Feb 8;2:14. doi: 10.3389/fmicb.2011.00014. eCollection 2011. Front Microbiol. 2011. PMID: 21687409 Free PMC article.
References
-
- Bannantine, J. P., R. S. Griffiths, W. Viratyosin, W. J. Brown, and D. D. Rockey. 2000. A secondary structure motif predictive of protein localization to the chlamydial inclusion membrane. Cell. Microbiol. 2:35-47. - PubMed
-
- Birkelund, S., L. Bini, V. Pallini, M. Sanchez-Campillo, S. Liberatori, J. D. Clausen, S. Ostergaard, A. Holm, and G. Christiansen. 1997. Characterization of Chlamydia trachomatis l2-induced tyrosine-phosphorylated HeLa cell proteins by two-dimensional gel electrophoresis. Electrophoresis 18:563-567. - PubMed
-
- Boleti, H., D. M. Ojcius, and A. Dautry-Varsat. 2000. Fluorescent labelling of intracellular bacteria in living host cells. J. Microbiol. Methods 40:265-274. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

