Listeriolysin O-induced membrane permeation mediates persistent interleukin-6 production in Caco-2 cells during Listeria monocytogenes infection in vitro
- PMID: 15972472
- PMCID: PMC1168588
- DOI: 10.1128/IAI.73.7.3869-3877.2005
Listeriolysin O-induced membrane permeation mediates persistent interleukin-6 production in Caco-2 cells during Listeria monocytogenes infection in vitro
Abstract
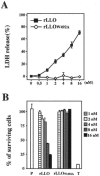
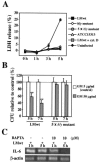
Listeriolysin O (LLO), a major virulence factor of Listeria monocytogenes, is a member of the cholesterol-dependent cytolysin family and plays important roles not only in survival of this bacterium in phagocytes but also in induction of various cellular responses, including cytokine production. In this work, we examined the involvement of LLO in induction of the cytokine response in intestinal epithelial cells, the front line of host defense against food-borne listeriosis. Infection of Caco-2 cells with wild-type L. monocytogenes induced persistent expression of interleukin-6 (IL-6) mRNA. In contrast, IL-6 expression was observed only transiently during infection with non-LLO-producing strains. A sublytic dose of recombinant LLO (rLLO) induced the expression of IL-6 via formation of membrane pores. Under conditions of LLO-induced pore formation without extensive cell lysis, Ca2+ influx was observed, and the IL-6 expression induced by rLLO was inhibited by pretreatment with 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM), an intracellular Ca2+ chelator. LLO secreted by cytoplasmic L. monocytogenes appeared to induce pore formation in the membrane and to enable the trafficking of intracellular and extracellular molecules. Pretreatment with BAPTA-AM inhibited persistent IL-6 expression in Caco-2 cells infected with wild-type L. monocytogenes. These results suggest that LLO is involved in IL-6 production in the late phase of infection through the formation of Ca2+-permeable pores and subsequent Ca2+-dependent modulation of signaling and gene expression.
Figures



Similar articles
-
Multifaceted activity of listeriolysin O, the cholesterol-dependent cytolysin of Listeria monocytogenes.Subcell Biochem. 2014;80:161-95. doi: 10.1007/978-94-017-8881-6_9. Subcell Biochem. 2014. PMID: 24798012 Free PMC article. Review.
-
Listeriolysin O-dependent bacterial entry into the cytoplasm is required for calpain activation and interleukin-1 alpha secretion in macrophages infected with Listeria monocytogenes.Infect Immun. 2010 May;78(5):1884-94. doi: 10.1128/IAI.01143-09. Epub 2010 Mar 1. Infect Immun. 2010. PMID: 20194588 Free PMC article.
-
Dependency of caspase-1 activation induced in macrophages by Listeria monocytogenes on cytolysin, listeriolysin O, after evasion from phagosome into the cytoplasm.J Immunol. 2008 Jun 15;180(12):7859-68. doi: 10.4049/jimmunol.180.12.7859. J Immunol. 2008. PMID: 18523249
-
Cytolysin-dependent escape of the bacterium from the phagosome is required but not sufficient for induction of the Th1 immune response against Listeria monocytogenes infection: distinct role of Listeriolysin O determined by cytolysin gene replacement.Infect Immun. 2007 Aug;75(8):3791-801. doi: 10.1128/IAI.01779-06. Epub 2007 May 21. Infect Immun. 2007. PMID: 17517863 Free PMC article.
-
Listeriolysin O: a phagosome-specific lysin.Microbes Infect. 2007 Aug;9(10):1176-87. doi: 10.1016/j.micinf.2007.05.005. Epub 2007 May 7. Microbes Infect. 2007. PMID: 17720603 Review.
Cited by
-
Pathogenic Biohacking: Induction, Modulation and Subversion of Host Transcriptional Responses by Listeria monocytogenes.Toxins (Basel). 2020 May 5;12(5):294. doi: 10.3390/toxins12050294. Toxins (Basel). 2020. PMID: 32380645 Free PMC article. Review.
-
Multifaceted activity of listeriolysin O, the cholesterol-dependent cytolysin of Listeria monocytogenes.Subcell Biochem. 2014;80:161-95. doi: 10.1007/978-94-017-8881-6_9. Subcell Biochem. 2014. PMID: 24798012 Free PMC article. Review.
-
Activation of cytosolic phospholipase A2alpha in resident peritoneal macrophages by Listeria monocytogenes involves listeriolysin O and TLR2.J Biol Chem. 2008 Feb 22;283(8):4744-55. doi: 10.1074/jbc.M709956200. Epub 2007 Dec 14. J Biol Chem. 2008. PMID: 18083708 Free PMC article.
-
Cellular functions and X-ray structure of anthrolysin O, a cholesterol-dependent cytolysin secreted by Bacillus anthracis.J Biol Chem. 2009 May 22;284(21):14645-56. doi: 10.1074/jbc.M807631200. Epub 2009 Mar 23. J Biol Chem. 2009. PMID: 19307185 Free PMC article.
-
Listeriolysin O Affects the Permeability of Caco-2 Monolayer in a Pore-Dependent and Ca2+-Independent Manner.PLoS One. 2015 Jun 18;10(6):e0130471. doi: 10.1371/journal.pone.0130471. eCollection 2015. PLoS One. 2015. PMID: 26087154 Free PMC article.
References
-
- Abreu, M. T., P. Vora, E. Faure, L. S. Thomas, E. T. Arnold, and M. Arditi. 2001. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J. Immunol. 167:1609-1616. - PubMed
-
- Chamaillard, M., M. Hashimoto, Y. Horie, J. Masumoto, S. Qiu, L. Saab, Y. Ogura, A. Kawasaki, K. Fukase, S. Kusumoto, M. A. Valvano, S. J. Foster, T. W. Mak, G. Nunez, and N. Inohara. 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4:702-707. - PubMed
-
- Chico-Calero, I., M. Suarez, B. Gonzalez-Zorn, M. Scortti, J. Slaghuis, W. Goebel, and J. A. Vazquez-Boland. 2002. Hpt, a bacterial homolog of the microsomal glucose-6-phosphate translocase, mediates rapid intracellular proliferation in Listeria. Proc. Natl. Acad. Sci. USA 99:431-436. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

