Intranasal administration of recombinant Neisseria gonorrhoeae transferrin binding proteins A and B conjugated to the cholera toxin B subunit induces systemic and vaginal antibodies in mice
- PMID: 15972481
- PMCID: PMC1168620
- DOI: 10.1128/IAI.73.7.3945-3953.2005
Intranasal administration of recombinant Neisseria gonorrhoeae transferrin binding proteins A and B conjugated to the cholera toxin B subunit induces systemic and vaginal antibodies in mice
Abstract
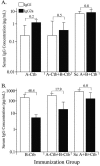
The transferrin binding proteins (TbpA and TbpB) comprise the gonococcal transferrin receptor and are considered potential antigens for inclusion in a vaccine against Neisseria gonorrhoeae. Intranasal (IN) immunization has shown promise in development of immunity against sexually transmitted disease pathogens, in part due to the induction of antigen-specific genital tract immunoglobulin A (IgA) and IgG. Conjugation of antigens to the highly immunogenic cholera toxin B subunit (Ctb) enhances antibody responses in the serum and mucosal secretions following IN vaccination. In the current study, we characterized the anti-Tbp immune responses following immunization of mice IN with recombinant transferrin binding proteins (rTbpA and rTbpB) conjugated to rCtb. We found that both rTbpA-Ctb and rTbpB-Ctb conjugates administered IN induced antibody responses in the serum and genital tract. IN immunization resulted in both IgA and IgG in the genital tract; however, subcutaneous immunization mainly generated IgG. Surprisingly, rTbpA alone was immunogenic and induced serum and mucosal antibody responses similar to those elicited against the rTbpA-Ctb conjugate. Overall, rTbpB was much more immunogenic than rTbpA, generating serum IgG levels that were greater than those elicited against rTbpA. Bactericidal assays conducted with sera collected from mice immunized IN with TbpA and/or TbpB indicated that both antigens generated antibodies with bactericidal activity. Anti-TbpA antibodies were cross-bactericidal against heterologous gonococcal strains, whereas TbpB-specific antibodies were less cross-reactive. By contrast, antibodies elicited via subcutaneous immunization were not cross-bactericidal against heterologous strains, indicating that IN vaccination could be the preferred route for elicitation of biologically functional antibodies.
Figures


Similar articles
-
Systemic and mucosal immune responses in mice after mucosal immunization with group B streptococcus type III capsular polysaccharide-cholera toxin B subunit conjugate vaccine.Infect Immun. 2000 Oct;68(10):5749-55. doi: 10.1128/IAI.68.10.5749-5755.2000. Infect Immun. 2000. PMID: 10992481 Free PMC article.
-
Vaccination of mice with gonococcal TbpB expressed in vivo from Venezuelan equine encephalitis viral replicon particles.Infect Immun. 2006 Mar;74(3):1612-20. doi: 10.1128/IAI.74.3.1612-1620.2006. Infect Immun. 2006. PMID: 16495532 Free PMC article.
-
The transferrin binding protein B of Moraxella catarrhalis elicits bactericidal antibodies and is a potential vaccine antigen.Infect Immun. 1998 Sep;66(9):4183-92. doi: 10.1128/IAI.66.9.4183-4192.1998. Infect Immun. 1998. PMID: 9712766 Free PMC article.
-
Mucosal immunity in the genital tract: prospects for vaccines against sexually transmitted diseases--a review.Am J Reprod Immunol. 1999 Jul;42(1):58-63. doi: 10.1111/j.1600-0897.1999.tb00466.x. Am J Reprod Immunol. 1999. PMID: 10429768 Review.
-
Mucosal vaccines based on the use of cholera toxin B subunit as immunogen and antigen carrier.Dev Biol Stand. 1994;82:215-27. Dev Biol Stand. 1994. PMID: 7958476 Review.
Cited by
-
Vaccines for gonorrhea: can we rise to the challenge?Front Microbiol. 2011 Jun 3;2:124. doi: 10.3389/fmicb.2011.00124. eCollection 2011. Front Microbiol. 2011. PMID: 21687431 Free PMC article.
-
Point Mutations in TbpA Abrogate Human Transferrin Binding in Neisseria gonorrhoeae.Infect Immun. 2022 Nov 17;90(11):e0041422. doi: 10.1128/iai.00414-22. Epub 2022 Nov 2. Infect Immun. 2022. PMID: 36321833 Free PMC article.
-
Microencapsulated IL-12 Drives Genital Tract Immune Responses to Intranasal Gonococcal Outer Membrane Vesicle Vaccine and Induces Resistance to Vaginal Infection with Diverse Strains of Neisseria gonorrhoeae.mSphere. 2023 Feb 21;8(1):e0038822. doi: 10.1128/msphere.00388-22. Epub 2022 Dec 20. mSphere. 2023. PMID: 36537786 Free PMC article.
-
TonB-Dependent Transporters Expressed by Neisseria gonorrhoeae.Front Microbiol. 2011 May 27;2:117. doi: 10.3389/fmicb.2011.00117. eCollection 2011. Front Microbiol. 2011. PMID: 21747812 Free PMC article.
-
Epidemiology, Treatments, and Vaccine Development for Antimicrobial-Resistant Neisseria gonorrhoeae: Current Strategies and Future Directions.Drugs. 2021 Jul;81(10):1153-1169. doi: 10.1007/s40265-021-01530-0. Epub 2021 Jun 7. Drugs. 2021. PMID: 34097283 Free PMC article. Review.
References
-
- Ahsan, F., J. J. Arnold, E. Meezan, and D. J. Pillion. 2001. Mutual inhibition of the insulin absorption-enhancing properties of dodecylmaltoside and dimethyl-beta-cyclodextrin following nasal administration. Pharm. Res. 18:608-614. - PubMed
-
- Arigita, C., G. F. Kersten, T. Hazendonk, W. E. Hennink, D. J. Crommelin, and W. Jiskoot. 2003. Restored functional immunogenicity of purified meningococcal PorA by incorporation into liposomes. Vaccine 21:950-960. - PubMed
-
- Boslego, J. W., E. C. Tramont, R. C. Chung, D. G. McChesney, J. Ciak, J. C. Sadoff, M. V. Piziak, J. D. Brown, C. C. Brinton, Jr., S. W. Wood, et al. 1991. Efficacy trial of a parenteral gonococcal pilus vaccine in men. Vaccine 9:154-162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

