Characterization of a human monoclonal antibody against Shiga toxin 2 expressed in Chinese hamster ovary cells
- PMID: 15972493
- PMCID: PMC1168570
- DOI: 10.1128/IAI.73.7.4054-4061.2005
Characterization of a human monoclonal antibody against Shiga toxin 2 expressed in Chinese hamster ovary cells
Abstract
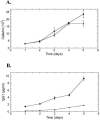
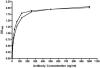
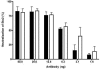
Shiga toxin-producing Escherichia coli infections can often lead to the development of hemolytic-uremic syndrome (HUS) in a small percentage of infected humans. Patients with HUS receive only supportive treatment as the benefit of antibiotic therapy remains uncertain. We have previously reported the generation and preclinical evaluation of neutralizing human monoclonal antibodies (HuMAbs) against the Shiga toxins (Stx). In this paper, we describe the expression in Chinese hamster ovary (CHO) cells of 5C12 HuMAb, which is directed against the A subunit of Stx2. The cDNAs of the light and heavy chain immunoglobulin (Ig) variable regions of 5C12 HuMAb were isolated and cloned into an expression vector containing human IgG1 constant regions. The vector was transfected into CHO cells, and transfectants secreting Stx2-specific antibody were screened by an Stx2-specific enzyme-linked immunosorbent assay. The CHO-produced recombinant 5C12 (r5C12) showed similar specificity and binding affinity to Stx2 as the parent hybridoma-produced 5C12. More significantly, the r5C12 displayed the same neutralizing activity as the parent 5C12 in vitro and in vivo. In the mouse toxicity model, both antibodies significantly and equally prolonged survival at a dose of 0.312 microg/mouse. The data showed that since r5C12, produced in CHO cells, was equally effective as the parent 5C12, it is our choice candidate as a potential prophylactic or therapeutic agent against hemolytic-uremic syndrome.
Figures

References
-
- Alt, F. W., R. E. Kellems, J. R. Bertino, and R. T. Schimke. 1978. Selective amplification of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J. Biol. Chem. 252:1357-1370. - PubMed
-
- Andersen, D. C., and L. Krummen. 2002. Recombinant protein expression for therapeutic applications. Curr. Opin. Biotechnol. 13:117-123. - PubMed
-
- Armstrong, G. D., P. C. Rowe, P. Goodyer, E. Orrbine, T. P. Klassen, G. Wells, A. MacKenzie, H. Lior, C. Blanchard, F. Auclair, et al. 1995. A phase I study of chemically synthesized verotoxin (Shiga-like toxin) Pk-trisaccharide receptors attached to chromosorb for preventing hemolytic-uremic syndrome. J. Infect. Dis. 171:1042-1045. - PubMed
-
- Arunachalam, B., S. Ghosh, G. P. Talwar, and R. Raghupathy. 1992. A single human monoclonal antibody that confers total protection from tetanus. Hybridoma 11:165-179. - PubMed
-
- Banatvala, N. P., P. M. Griffin, K. D. Greene, T. J. Barrett, W. F. Bibb, J. H. Green, and J. G. Wells. 2001. The United States National Prospective Hemolytic Uremic Syndrome Study: microbiologic, serologic, clinical, and epidemiologic findings. J. Infect. Dis. 183:1063-1070. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

