Characterization of native outer membrane vesicles from lpxL mutant strains of Neisseria meningitidis for use in parenteral vaccination
- PMID: 15972495
- PMCID: PMC1168616
- DOI: 10.1128/IAI.73.7.4070-4080.2005
Characterization of native outer membrane vesicles from lpxL mutant strains of Neisseria meningitidis for use in parenteral vaccination
Abstract
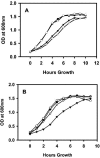
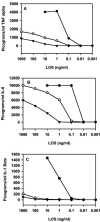
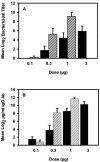
Native outer membrane vesicles (NOMV) of Neisseria meningitidis consist of intact outer membrane and contain outer membrane proteins (OMP) and lipooligosaccharides (LOS) in their natural conformation and membrane environment. NOMV have been safely used intranasally in P1 studies with encouraging results, but they are too toxic for parenteral vaccination. We now report the preparation and characterization of lpxL mutants that express LOS with reduced toxicity, and the evaluation of the potential of NOMV from these strains for use as a parenteral vaccine. A series of deletion mutants were prepared with knockouts of one or more of the lpxL1, lpxL2, or synX genes. The deltalpxL2 mutants had a reduced growth rate, reduced level of LOS expression, and increased sensitivity to surfactants. In addition, deltasynX deltalpxL2 double mutants had reduced viability in stationary phase. The deltalpxL1 deltalpxL2 double mutant behaved essentially the same as the deltalpxL2 single mutant. LOS from both lpxL mutant strains exhibited altered migration on polyacrylamide gels. The LOS of deltalpxL2 mutants of L3,7 strains were fully sialylated. NOMV prepared from lpxL2 mutants was about 200-fold less active than wild-type NOMV in rabbit pyrogen tests and in tumor necrosis factor alpha release assays. Bactericidal titers induced in animals by deltalpxL2 mutant NOMV were lower than those induced by deltalpxL1 or wild-type NOMV. However, immunogenicity could be largely restored by use of an adjuvant. These results provide evidence that NOMV from deltalpxL2 mutant strains will be safe and immunogenic in humans when given parenterally.
Figures
References
-
- Bjune, G., E. A. Høiby J. K. Grønnesby, (swsl)O. Arnesen, J. H. Fredriksen, A. Halstensen, E. Holten, A. K. Lindbak, H. Nøkleby, E. Rosenqvist, L. K. Solberg, O. Closs, J. Eng, L. O. Frøholm, A. Lystad, L. S. Bakketeig, and B. Hareide. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093-1096. - PubMed
-
- Boslego, J., J. Garcia, C. Cruz, W. Zollinger, B. Brandt, S. Ruiz, M. Martinez, J. Arthur, P. Underwood, W. Silva, et al. 1995. Efficacy, safety, and immunogenicity of a meningococcal group B (15:P1.3) outer membrane protein vaccine in Iquique, Chile. Vaccine 13:821-829. - PubMed
-
- Bruge, J., N. Bouveret-Le Cam, B. Danve, G. Rougon, and D. Schulz. 2004. Clinical evaluation of a group B meningococcal N-propionylated polysaccharide conjugate vaccine in adult, male volunteers. Vaccine 22:1087-1096. - PubMed
-
- Cartwright, K., R. Morris, H. Rumke, A. Fox, R. Borrow, N. Begg, P. Richmond, and J. Poolman. 1999. Immunogenicity and reactogenicity in UK infants of a novel meningococcal vesicle vaccine containing multiple class 1 (PorA) outer membrane proteins. Vaccine 17:2612-2619. - PubMed
-
- Catlin, B. W. 1973. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J. Infect. Dis. 128:178-194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

