Superoxide production in Galleria mellonella hemocytes: identification of proteins homologous to the NADPH oxidase complex of human neutrophils
- PMID: 15972506
- PMCID: PMC1168619
- DOI: 10.1128/IAI.73.7.4161-4170.2005
Superoxide production in Galleria mellonella hemocytes: identification of proteins homologous to the NADPH oxidase complex of human neutrophils
Abstract
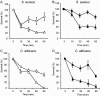
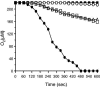
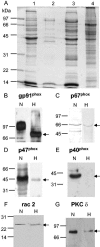
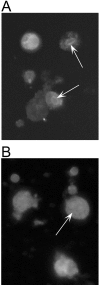
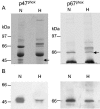
The insect immune response has a number of structural and functional similarities to the innate immune response of mammals. The objective of the work presented here was to establish the mechanism by which insect hemocytes produce superoxide and to ascertain whether the proteins involved in superoxide production are similar to those involved in the NADPH oxidase-induced superoxide production in human neutrophils. Hemocytes of the greater wax moth (Galleria mellonella) were shown to be capable of phagocytosing bacterial and fungal cells. The kinetics of phagocytosis and microbial killing were similar in the insect hemocytes and human neutrophils. Superoxide production and microbial killing by both cell types were inhibited in the presence of the NADPH oxidase inhibitor diphenyleneiodonium chloride. Immunoblotting of G. mellonella hemocytes with antibodies raised against human neutrophil phox proteins revealed the presence of proteins homologous to gp91phox, p67phox, p47phox, and the GTP-binding protein rac 2. A protein equivalent to p40phox was not detected in insect hemocytes. Immunofluorescence analysis localized insect 47-kDa and 67-kDa proteins throughout the cytosol and in the perinuclear region. Hemocyte 67-kDa and 47-kDa proteins were immunoprecipitated and analyzed by matrix-assisted laser desorption ionization--time of flight analysis. The results revealed that the hemocyte 67-kDa and 47-kDa proteins contained peptides matching those of p67phox and p47phox of human neutrophils. The results presented here indicate that insect hemocytes phagocytose and kill bacterial and fungal cells by a mechanism similar to the mechanism used by human neutrophils via the production of superoxide. We identified proteins homologous to a number of proteins essential for superoxide production in human neutrophils and demonstrated that significant regions of the 67-kDa and 47-kDa insect proteins are identical to regions of the p67phox and p47phox proteins of neutrophils.
Figures
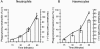


References
-
- Arumugam, M., B. Romestand, J. Torreilles, and P. Roch. 2000. In vitro production of superoxide and nitric oxide (as nitrite and nitrate) by Mytilus galloprovincialis haemocytes upon incubation with PMA or laminarin or during yeast phagocytosis. Eur. J. Cell Biol. 79:513-519. - PubMed
-
- Baggiolini, M., W. Ruch, and P. H. Cooper. 1986. Measurement of hydrogen peroxide production by phagocytes using homovanillic acid and horse radish peroxidase. Methods Enzymol. 132:395-400. - PubMed
-
- Balls, M. 1999. Science without guinea pigs. Res. Teach. Dev. Inf. 24:26-28.
-
- Banfi, B., R. A. Clark, K. Steger, and K. H. Krause. 2003. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J. Biol. Chem. 278:3510-3513. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

