Neisseria gonorrhoeae kills carcinoembryonic antigen-related cellular adhesion molecule 1 (CD66a)-expressing human B cells and inhibits antibody production
- PMID: 15972507
- PMCID: PMC1168567
- DOI: 10.1128/IAI.73.7.4171-4179.2005
Neisseria gonorrhoeae kills carcinoembryonic antigen-related cellular adhesion molecule 1 (CD66a)-expressing human B cells and inhibits antibody production
Abstract
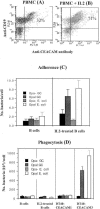
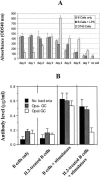
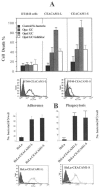
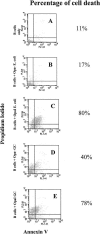
Neisseria gonorrhoeae cells (gonococci [GC]), the etiological agents for gonorrhea, can cause repeated infections. During and after gonococcal infection, local and systemic antigonococcal antibody levels are low. These clinical data indicate the possibility that GC may suppress immune responses during infection. Carcinoembryonic antigen-related cellular adhesion molecule 1 (CEACAM1 or CD66a), a receptor for GC opacity (Opa) proteins, was shown to mediate inhibitory signals. In the present study, human B cells were activated by interleukin-2 to express CEACAM1 and then stimulated to secrete antibodies and simultaneously coincubated with Opa- and OpaI GC of strain MS11. Our results show that this OpaI GC has the ability to inhibit antibody production. The interaction of GC and CEACAM1 with human peripheral B cells also results in induction of cell death. The same findings were observed in DT40 B cells. This CEACAM1-promoted cell death pathway does not involve the inhibitory signals or the tyrosine phosphatases SHP-1 and SHP-2 but depends on Bruton's tyrosine kinase in DT40 cells. Our results suggest that Neisseria gonorrhoeae possesses the ability to suppress antibody production by killing CEACAM1-expressing B cells.
Figures
References
-
- Beauchemin, N., T. Kunath, J. Robitaille, B. Chow, C. Turbide, E. Daniels, and A. Veillette. 1997. Association of biliary glycoprotein with protein tyrosine phosphatase SHP-1 in malignant colon epithelial cells. Oncogene 14:783-790. - PubMed
-
- Belland, R. J., T. Chen, J. Swanson, and S. H. Fischer. 1992. Human neutrophil response to recombinant neisserial Opa proteins. Mol. Microbiol. 6:1729-1737. - PubMed
-
- Belland, R. J., S. G. Morrison, P. van der Ley, and J. Swanson. 1989. Expression and phase variation of gonococcal P.II genes in Escherichia coli involves ribosomal frameshifting and slipped-strand mispairing. Mol. Microbiol. 3:777-786. - PubMed
-
- Bhat, K. S., C. P. Gibbs, O. Barrera, S. G. Morrison, F. Jahnig, A. Stern, E.-M. Kupsch, T. F. Meyer, and J. Swanson. 1991. The opacity proteins of Neisseria gonorrhoeae strain MS11 are encoded by a family of 11 complete genes. Mol. Microbiol. 5:1889-1901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

