Impact of Helicobacter pylori virulence factors and compounds on activation and maturation of human dendritic cells
- PMID: 15972508
- PMCID: PMC1168582
- DOI: 10.1128/IAI.73.7.4180-4189.2005
Impact of Helicobacter pylori virulence factors and compounds on activation and maturation of human dendritic cells
Abstract
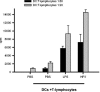
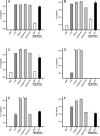
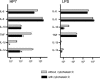
Recently, we and others have shown that Helicobacter pylori induces dendritic cell (DC) activation and maturation. However, the impact of virulence factors on the interplay between DCs and H. pylori remains elusive. Therefore, we investigated the contribution of cag pathogenicity island (PAI) and VacA status on cytokine release and up-regulation of costimulatory molecules in H. pylori-treated DCs. In addition, to characterize the stimulatory capacity of H. pylori compounds in more detail, we studied the effect of formalin-inactivated and sonicated H. pylori, as well as secreted H. pylori molecules, on DCs. Incubation of DCs with viable or formalin-inactivated H. pylori induced comparable secretion of interleukin-6 (IL-6), IL-8, IL-10, IL-12, IL-1beta, and tumor necrosis factor (TNF). In contrast, IL-12 and IL-1beta release was significantly reduced in DCs treated with sonicated bacteria and secreted bacterial molecules. Treatment of sonicated H. pylori preparations with polymyxin B resulted in a significant reduction of IL-8 and IL-6 secretion, suggesting that H. pylori-derived lipopolysaccharide at least partially contributes to activation of immature DCs. In addition, the capacity of H. pylori-pulsed DCs to activate allogeneic T cells was not affected by cag PAI and VacA. Pretreatment of DC with cytochalasin D significantly inhibited secretion of IL-12, IL-1beta, and TNF, indicating that phagocytosis of H. pylori contributes to maximal activation of DCs. Taken together, our results suggest that DC activation and maturation, as well as DC-mediated T-cell activation, are independent of the cag PAI and VacA status of H. pylori.
Figures



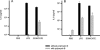
References
-
- Akanuma, M., S. Maeda, K. Ogura, Y. Mitsuno, Y. Hirata, T. Ikenoue, M. Otsuka, T. Watanabe, Y. Yamaji, H. Yoshida, T. Kawabe, Y. Shiratori, and M. Omata. 2002. The evaluation of putative virulence factors of Helicobacter pylori for gastroduodenal disease by use of a short-term Mongolian gerbil infection model. J. Infect. Dis. 185:341-347. - PubMed
-
- Allen, L. A. 2003. Mechanisms of pathogenesis: evasion of killing by polymorphonuclear leukocytes. Microbes Infect. 5:1329-1335. - PubMed
-
- Asaka, M., M. Kudo, M. Kato, T. Kimura, T. Meguro, S. Mitani, T. Miyazaki, and K. Inoue. 1994. The role of Helicobacter pylori infection in the pathogenesis of gastritis. J. Gastroenterol. 29(Suppl. 7):100-104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

