Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function in heart failure
- PMID: 15972811
- PMCID: PMC1172237
- DOI: 10.1073/pnas.0500353102
Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function in heart failure
Abstract
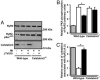
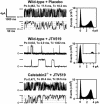
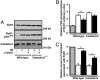
Abnormalities in intracellular calcium release and reuptake are responsible for decreased contractility in heart failure (HF). We have previously shown that cardiac ryanodine receptors (RyRs) are protein kinase A-hyperphosphorylated and depleted of the regulatory subunit calstabin-2 in HF. Moreover, similar alterations in skeletal muscle RyR have been linked to increased fatigability in HF. To determine whether restoration of calstabin binding to RyR may ameliorate cardiac and skeletal muscle dysfunction in HF, we treated WT and calstabin-2-/- mice subjected to myocardial infarction (MI) with JTV519. JTV519, a 1,4-benzothiazepine, is a member of a class of drugs known as calcium channel stabilizers, previously shown to increase calstabin binding to RyR. Echocardiography at 21 days after MI demonstrated a significant increase in ejection fraction in WT mice treated with JTV519 (45.8 +/- 5.1%) compared with placebo (31.1 +/- 3.1%; P < 0.05). Coimmunoprecipitation experiments revealed increased amounts of calstabin-2 bound to the RyR2 channel in JTV519-treated WT mice. However, JTV519 did not show any of these beneficial effects in calstabin-2-/- mice with MI. Additionally, JTV519 improved skeletal muscle fatigue in WT and calstabin-2-/- mice with HF by increasing the binding of calstabin-1 to RyR1. The observation that treatment with JTV519 improved cardiac function in WT but not calstabin-2-/- mice indicates that calstabin-2 binding to RyR2 is required for the beneficial effects in failing hearts. We conclude that JTV519 may provide a specific way to treat the cardiac and skeletal muscle myopathy in HF by increasing calstabin binding to RyR.
Figures


References
-
- Hunt, S. A., Baker, D. W., Chin, M. H., Cinquegrani, M. P., Feldman, A. M., Francis, G. S., Ganiats, T. G., Goldstein, S., Gregoratos, G., Jessup, M. L., et al. (2001) J. Am. Coll. Cardiol. 38, 2101-2113. - PubMed
-
- Kramer, R. S., Mason, D. T. & Braunwald, E. (1968) Circulation 38, 629-634. - PubMed
-
- Harrington, D. & Coats, A. J. (1997) Curr. Opin. Cardiol. 12, 224-232. - PubMed
-
- Bers, D. M., Eisner, D. A. & Valdivia, H. H. (2003) Circ. Res. 93, 487-490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

