Hysteresis in a synthetic mammalian gene network
- PMID: 15972812
- PMCID: PMC1172236
- DOI: 10.1073/pnas.0500345102
Hysteresis in a synthetic mammalian gene network
Abstract
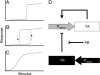
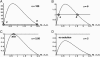
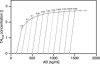
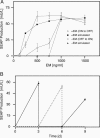
Bistable and hysteretic switches, enabling cells to adopt multiple internal expression states in response to a single external input signal, have a pivotal impact on biological systems, ranging from cell-fate decisions to cell-cycle control. We have designed a synthetic hysteretic mammalian transcription network. A positive feedback loop, consisting of a transgene and transactivator (TA) cotranscribed by TA's cognate promoter, is repressed by constitutive expression of a macrolide-dependent transcriptional silencer, whose activity is modulated by the macrolide antibiotic erythromycin. The antibiotic concentration, at which a quasi-discontinuous switch of transgene expression occurs, depends on the history of the synthetic transcription circuitry. If the network components are imbalanced, a graded rather than a quasi-discontinuous signal integration takes place. These findings are consistent with a mathematical model. Synthetic gene networks, which are able to emulate natural gene expression behavior, may foster progress in future gene therapy and tissue engineering initiatives.
Figures

Similar articles
-
Artificial regulatory networks and cascades for discrete multilevel transgene control in mammalian cells.Biotechnol Bioeng. 2003 Sep 30;83(7):810-20. doi: 10.1002/bit.10731. Biotechnol Bioeng. 2003. PMID: 12889021
-
Macrolide-based transgene control in mammalian cells and mice.Nat Biotechnol. 2002 Sep;20(9):901-7. doi: 10.1038/nbt731. Epub 2002 Aug 19. Nat Biotechnol. 2002. PMID: 12205509
-
Improved transgene expression fine-tuning in mammalian cells using a novel transcription-translation network.J Biotechnol. 2006 Aug 5;124(4):732-46. doi: 10.1016/j.jbiotec.2006.01.003. Epub 2006 Feb 20. J Biotechnol. 2006. PMID: 16488500
-
Mammalian synthetic biology: engineering of sophisticated gene networks.J Biotechnol. 2007 Jul 15;130(4):329-45. doi: 10.1016/j.jbiotec.2007.05.014. Epub 2007 May 24. J Biotechnol. 2007. PMID: 17602777 Review.
-
Recent advances in mammalian synthetic biology-design of synthetic transgene control networks.Curr Opin Biotechnol. 2009 Aug;20(4):449-60. doi: 10.1016/j.copbio.2009.07.009. Epub 2009 Sep 15. Curr Opin Biotechnol. 2009. PMID: 19762224 Review.
Cited by
-
Biofunctionalized Materials Featuring Feedforward and Feedback Circuits Exemplified by the Detection of Botulinum Toxin A.Adv Sci (Weinh). 2018 Nov 28;6(4):1801320. doi: 10.1002/advs.201801320. eCollection 2019 Feb 20. Adv Sci (Weinh). 2018. PMID: 30828524 Free PMC article.
-
DNA looping in prokaryotes: experimental and theoretical approaches.J Bacteriol. 2013 Mar;195(6):1109-19. doi: 10.1128/JB.02038-12. Epub 2013 Jan 4. J Bacteriol. 2013. PMID: 23292776 Free PMC article. Review.
-
Load-Dependent Changes in Left Ventricular Structure and Function in a Pathophysiologically Relevant Murine Model of Reversible Heart Failure.Circ Heart Fail. 2018 May;11(5):e004351. doi: 10.1161/CIRCHEARTFAILURE.117.004351. Circ Heart Fail. 2018. PMID: 29716898 Free PMC article.
-
Engineering of a synthetic quadrastable gene network to approach Waddington landscape and cell fate determination.Elife. 2017 Apr 11;6:e23702. doi: 10.7554/eLife.23702. Elife. 2017. PMID: 28397688 Free PMC article.
-
Multi-chromatic control of mammalian gene expression and signaling.Nucleic Acids Res. 2013 Jul;41(12):e124. doi: 10.1093/nar/gkt340. Epub 2013 Apr 26. Nucleic Acids Res. 2013. PMID: 23625964 Free PMC article.
References
-
- Ball, P. (2004) Critical Mass: How One Thing Leads to Another (William Heinemann, London).
-
- Ludwig, D., Jones, D. D. & Holling, C. S. (1978) J. Anim. Ecol. 47, 315-332.
-
- Bren, A. & Eisenbach, M. (2001) J. Mol. Biol. 312, 699-709. - PubMed
-
- Barrett, J. (2001) Int. J. Biochem. Cell Biol. 33, 105-117. - PubMed
-
- Ozbudak, E. M., Thattai, M., Lim, H. N., Shraiman, B. I. & Van Oudenaarden, A. (2004) Nature 427, 737-740. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

