Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria
- PMID: 15972817
- PMCID: PMC4113954
- DOI: 10.1074/jbc.M504955200
Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria
Abstract
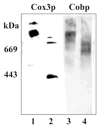
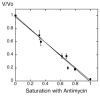
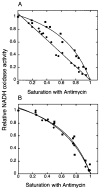
Digitonin extracts of mitochondria from cardiolipin-containing (wild type) and cardiolipin-lacking (crd1Delta mutant) Saccharomyces cerevisiae subjected to colorless native polyacrylamide gel electrophoresis in the presence of 0.003% digitonin displayed a supercomplex composed of homodimers of complexes III and IV in the former case but only the individual homodimers in the latter case. To avoid treatment with any detergent or dye, we compared organization of the respiratory chain in intact mitochondria from wild type and cardiolipin-lacking cells by using a functional analysis developed previously for the study of the organization of the respiratory chain of S. cerevisiae (Boumans, H., Grivell, L. A., and Berden, J. A. (1998) J. Biol. Chem. 273, 4872-4877). Dependence of the kinetics of NADH oxidation via complexes III, IV, and cytochrome c on the concentration of the complex III-specific inhibitor antimycin A was studied. A linear relationship between respiratory activity and saturation of complex III with antimycin A was obtained for wild type mitochondria consistent with single functional unit kinetics of the respiratory chain. Under the same conditions, cardiolipin-lacking mitochondria displayed a hyperbolic relationship indicating cytochrome c pool behavior. No release of cytochrome c from cardiolipin-lacking mitochondria or mitoplasts under our standard experimental conditions was detected. Identical cytochrome c pool behavior was observed for both wild type and cardiolipin-lacking mitochondria in the presence of a chaotropic agent, which disrupts the interaction between respiratory complexes. The results demonstrate that cardiolipin is essential for association of complexes III and IV into a supercomplex in intact yeast mitochondria.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

