X-linked anhidrotic ectodermal dysplasia disruption yields a mouse model for ocular surface disease and resultant blindness
- PMID: 15972955
- PMCID: PMC1603450
- DOI: 10.1016/S0002-9440(10)62956-2
X-linked anhidrotic ectodermal dysplasia disruption yields a mouse model for ocular surface disease and resultant blindness
Abstract
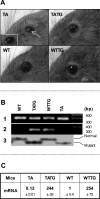
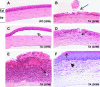
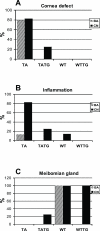
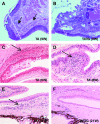
X-linked anhidrotic/hypohidrotic ectodermal dysplasia (EDA) is caused by mutations in the (EDA) gene, which is required for the morphogenesis of ectoderm-derived tissues. Although EDA function in skin appendage development has been studied in Eda mutant "Tabby" mice, we have recently identified characteristic abnormalities in the ocular surface, an ectoderm-derived tissue. Histology of eyes of Tabby males revealed that 1) as previously reported, mice lacked meibomian glands; 2) >80% developed corneal lesions such as neovascularization, keratitis, ulceration, and keratinization identifiable from 9 weeks of age; and 3) > 80% showed ocular surface inflammation (blepharitis and conjunctivitis) when housed in a standard environment. Strikingly, both corneal defects and inflammation were prevented in Tabby mice bearing a transgene for the Eda-A1 isoform, but meibomian glands were restored little if at all. These findings suggest that intact ocular surface health is EDA dependent and that Tabby corneal abnormalities are not solely dependent on meibomian gland lipid secretion. Alternatively, susceptibility to inflammation and other phenotypes could result from failure of the usual EDA receptor to activate nuclear factor-kappaB transcription factors. This can be further tested in Tabby and Tabby-EDA transgenic mice, which provide unique models of severe ocular surface disease.
Figures


Similar articles
-
Ectodysplasin A protein promotes corneal epithelial cell proliferation.J Biol Chem. 2017 Aug 11;292(32):13391-13401. doi: 10.1074/jbc.M117.803809. Epub 2017 Jun 27. J Biol Chem. 2017. PMID: 28655773 Free PMC article.
-
Meibomian Gland Absence Related Dry Eye in Ectodysplasin A Mutant Mice.Am J Pathol. 2016 Jan;186(1):32-42. doi: 10.1016/j.ajpath.2015.09.019. Epub 2015 Nov 25. Am J Pathol. 2016. PMID: 26626448
-
EDA targets revealed by skin gene expression profiles of wild-type, Tabby and Tabby EDA-A1 transgenic mice.Hum Mol Genet. 2002 Jul 15;11(15):1763-73. doi: 10.1093/hmg/11.15.1763. Hum Mol Genet. 2002. PMID: 12095918
-
Recent advances in understanding of the molecular basis of anhidrotic ectodermal dysplasia: discovery of a ligand, ectodysplasin A and its two receptors.J Appl Genet. 2002;43(1):97-107. J Appl Genet. 2002. PMID: 12084975 Review.
-
Hypohidrotic (anhidrotic) ectodermal dysplasia: molecular genetic research and its clinical applications.Semin Dermatol. 1993 Sep;12(3):241-6. Semin Dermatol. 1993. PMID: 8217562 Review.
Cited by
-
Unexplained Fever in Infancy: Report of a Rare Case of Hypohidrotic Ectodermal Dysplasia in an Infant.Cureus. 2023 May 25;15(5):e39489. doi: 10.7759/cureus.39489. eCollection 2023 May. Cureus. 2023. PMID: 37362526 Free PMC article.
-
Alkali burn injury model of meibomian gland dysfunction in mice.Int J Ophthalmol. 2024 Dec 18;17(12):2158-2166. doi: 10.18240/ijo.2024.12.02. eCollection 2024. Int J Ophthalmol. 2024. PMID: 39697880 Free PMC article.
-
A Novel Model of Meibomian Gland Dysfunction Induced with Complete Freund's Adjuvant in Rabbits.Vision (Basel). 2017 Feb 9;1(1):10. doi: 10.3390/vision1010010. Vision (Basel). 2017. PMID: 31740635 Free PMC article.
-
Extended Overview of Ocular Phenotype with Recent Advances in Hypohidrotic Ectodermal Dysplasia.Children (Basel). 2022 Sep 6;9(9):1357. doi: 10.3390/children9091357. Children (Basel). 2022. PMID: 36138666 Free PMC article. Review.
-
Requirement for Shh and Fox family genes at different stages in sweat gland development.Hum Mol Genet. 2009 May 15;18(10):1769-78. doi: 10.1093/hmg/ddp089. Epub 2009 Mar 6. Hum Mol Genet. 2009. PMID: 19270025 Free PMC article.
References
-
- Pinheiro M, Freire-Maia N. Ectodermal dysplasias: a clinical classification and a causal review. Am J Med Genet. 1994;53:153–162. - PubMed
-
- Priolo M, Silengo M, Lerone M, Ravazzolo R. Ectodermal dysplasias: not only ‘skin’ deep. Clin Genet. 2000;58:415–430. - PubMed
-
- Kere J, Srivastava AK, Montonen O, Zonana J, Thomas N, Ferguson B, Munoz F, Morgan D, Clarke A, Baybayan P, Chen EY, Ezer S, Saarialho-Kere U, de la Chapelle A, Schlessinger D. X-linked anhidrotic (hypohidrotic) ectodermal dysplasia is caused by mutation in a novel transmembrane protein. Nat Genet. 1996;13:409–416. - PubMed
-
- Srivastava AK, Pispa J, Hartung AJ, Du Y, Ezer S, Jenks T, Shimada T, Pekkanen M, Mikkola ML, Ko MS, Thesleff I, Kere J, Schlessinger D. The Tabby phenotype is caused by mutation in a mouse homologue of the EDA gene that reveals novel mouse and human exons and encodes a protein (ectodysplasin-A) with collagenous domains. Proc Natl Acad Sci USA. 1997;94:13069–13074. - PMC - PubMed
-
- Headon DJ, Overbeek PA. Involvement of a novel Tnf receptor homologue in hair follicle induction. Nat Genet. 1999;22:370–374. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

