Increased toll-like receptor 4 expression in thymus of myasthenic patients with thymitis and thymic involution
- PMID: 15972959
- PMCID: PMC1603452
- DOI: 10.1016/S0002-9440(10)62960-4
Increased toll-like receptor 4 expression in thymus of myasthenic patients with thymitis and thymic involution
Abstract
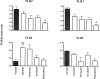
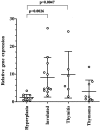
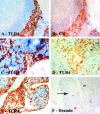
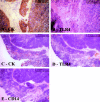
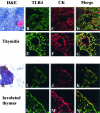
Thymic abnormalities are present in approximately 80% of myasthenia gravis (MG) patients, and the thymus seems to be the main site of autosensitization to the acetylcholine receptor. In view of findings that the innate immune system can generate an autoimmune response, we studied the expression of Toll-like receptors (TLRs) 2 to 5, key components of innate immunity signaling pathways, in 37 thymuses from patients with autoimmune MG. TLR4 mRNA levels were significantly greater in thymitis (hyperplasia with diffuse B-cell infiltration) and involuted thymus than in germinal center hyperplasia and thymoma. By immunohistochemistry and confocal microscopy, cells positive for TLR4 protein were rarely detected in thymoma. However, in thymitis TLR4 protein was mostly found on epitheliomorphic (cytokeratin-positive) cells located in close association with clusters of acetylcholine receptor-positive myoid cells in thymic medulla and also at the borders between cortical and medullary areas. B cells were never TLR4-positive. TLR4 protein was also present in remnant tissue of involuted thymus. This is the first finding of a possible link between innate immunity and MG. We speculate that in a subgroup of MG patients, an exogenous or endogenous danger signal may activate the innate immune system and give rise to TLR4-mediated mechanisms contributing to autoimmunity.
Figures


Similar articles
-
Increased expression of Toll-like receptors 7 and 9 in myasthenia gravis thymus characterized by active Epstein-Barr virus infection.Immunobiology. 2016 Apr;221(4):516-27. doi: 10.1016/j.imbio.2015.12.007. Epub 2015 Dec 12. Immunobiology. 2016. PMID: 26723518
-
Detection of poliovirus-infected macrophages in thymus of patients with myasthenia gravis.Neurology. 2010 Apr 6;74(14):1118-26. doi: 10.1212/WNL.0b013e3181d7d884. Neurology. 2010. PMID: 20368632
-
Innate immunity in myasthenia gravis thymus: pathogenic effects of Toll-like receptor 4 signaling on autoimmunity.J Autoimmun. 2014 Aug;52:74-89. doi: 10.1016/j.jaut.2013.12.013. Epub 2014 Jan 4. J Autoimmun. 2014. PMID: 24397961
-
Myasthenia gravis.Verh Dtsch Ges Pathol. 1996;80:116-26. Verh Dtsch Ges Pathol. 1996. PMID: 9020569 Review.
-
Thymus involvement in early-onset myasthenia gravis.Ann N Y Acad Sci. 2018 Jan;1412(1):137-145. doi: 10.1111/nyas.13519. Epub 2017 Nov 10. Ann N Y Acad Sci. 2018. PMID: 29125185 Review.
Cited by
-
Review on Toll-Like Receptor Activation in Myasthenia Gravis: Application to the Development of New Experimental Models.Clin Rev Allergy Immunol. 2017 Feb;52(1):133-147. doi: 10.1007/s12016-016-8549-4. Clin Rev Allergy Immunol. 2017. PMID: 27207173 Review.
-
The impact of the gut microbiota on T cell ontogeny in the thymus.Cell Mol Life Sci. 2022 Apr 4;79(4):221. doi: 10.1007/s00018-022-04252-y. Cell Mol Life Sci. 2022. PMID: 35377005 Free PMC article. Review.
-
Role of miRNAs in Normal and Myasthenia Gravis Thymus.Front Immunol. 2020 Jun 10;11:1074. doi: 10.3389/fimmu.2020.01074. eCollection 2020. Front Immunol. 2020. PMID: 32587589 Free PMC article. Review.
-
Inflammatory Role of TLR-MyD88 Signaling in Multiple Sclerosis.Front Mol Neurosci. 2020 Jan 10;12:314. doi: 10.3389/fnmol.2019.00314. eCollection 2019. Front Mol Neurosci. 2020. PMID: 31998072 Free PMC article.
-
Identification of novel MicroRNA signatures linked to experimental autoimmune myasthenia gravis pathogenesis: down-regulated miR-145 promotes pathogenetic Th17 cell response.J Neuroimmune Pharmacol. 2013 Dec;8(5):1287-302. doi: 10.1007/s11481-013-9498-9. Epub 2013 Sep 17. J Neuroimmune Pharmacol. 2013. PMID: 24043548
References
-
- Vincent A. Unravelling the pathogenesis of myasthenia gravis. Nat Rev Immunol. 2002;2:797–804. - PubMed
-
- Drachman DB. Myasthenia gravis. N Engl J Med. 1994;330:1797–1810. - PubMed
-
- Sommer N, Harcourt GC, Willcox N, Beeson D, Newsom-Davis J. Acetylcholine receptor-reactive T lymphocytes from healthy subjects and myasthenia gravis patients. Neurology. 1991;41:1270–1276. - PubMed
-
- Marx A, Wilisch A, Schultz A, Gattenlohner S, Nenninger R, Müller-Hermelink HK. Pathogenesis of myasthenia gravis. Virchows Arch. 1997;430:355–364. - PubMed
-
- Gronseth GS, Barohn RJ. Practice parameter: thymectomy for autoimmune myasthenia gravis (an evidence-based review). Neurology. 2000;55:7–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

