Perturbed neurogenesis in the adult hippocampus associated with presenilin-1 A246E mutation
- PMID: 15972961
- PMCID: PMC1603433
- DOI: 10.1016/S0002-9440(10)62962-8
Perturbed neurogenesis in the adult hippocampus associated with presenilin-1 A246E mutation
Abstract
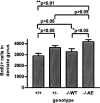
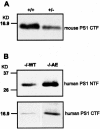
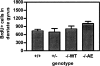
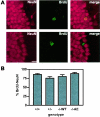
In addition to its well-established role in gamma-secretase cleavage, presenilin (PS) also plays a role in regulating the stability of cytosolic beta-catenin, a protein involved in Wnt signaling. Several familial Alzheimer's disease-associated PS1 mutations have been shown to increase the stability of the signaling pool of beta-catenin, correlating with enhanced cell proliferation. Accordingly, we hypothesized that in the setting of PS1 mutations, abnormal activation of Wnt/beta-catenin signaling leads to increased cell division. We tested this hypothesis by examining whether there is evidence of increased neurogenesis in the hippocampus of adult transgenic mice that overexpress the PS1 A246E mutation. In PS1/PS2-deficient fibroblasts, expression of PS1 A246E Familial AD mutation failed to restore the rapid turnover of beta-catenin compared with wild-type PS1. We then examined whether the same mutation enhanced neurogenesis in vivo in adult hippocampus of PS1-deficient mice when restored by wild-type human PS1 (PS1(-/-)WT) or A246E PS1 mutation (PS1(-/-)AE). The PS1 A246E mutation stimulated the proliferation of progenitor cells in the dentate gyrus of adult mice, as assessed by 5-bromo-2-deoxyuridine incorporation, but did not influence their survival or differentiation. These observations suggest that the PS1 A246E mutation influences cell growth putatively via abnormal beta-catenin signaling in vivo.
Figures


References
-
- St George-Hyslop PH. Molecular genetics of Alzheimer’s disease. Biol Psychiatry. 2000;47:183–199. - PubMed
-
- Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–713. - PubMed
-
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2:864–870. - PubMed
-
- Wen PH, Friedrich VL, Jr, Shioi J, Robakis NK, Elder GA. Presenilin-1 is expressed in neural progenitor cells in the hippocampus of adult mice. Neurosci Lett. 2002;318:53–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

