c-Fos-dependent induction of the small ras-related GTPase Rab11a in skin carcinogenesis
- PMID: 15972968
- PMCID: PMC1603444
- DOI: 10.1016/S0002-9440(10)62969-0
c-Fos-dependent induction of the small ras-related GTPase Rab11a in skin carcinogenesis
Abstract
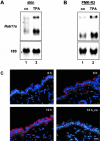
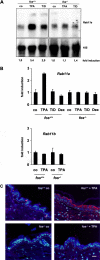
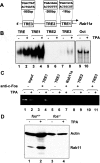
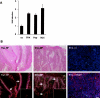
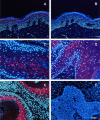
Malignant transformation of mouse skin by tumor promoters and chemical carcinogens, such as the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA), is a multistage process leading to the formation of squamous cell carcinomas. It has been shown that mice lacking the AP-1 family member c-Fos exhibit an impaired transition from benign to malignant skin tumors. Here, we demonstrate enhanced expression of the small Ras-related GTPase Rab11a after short-term TPA treatment of mouse back skin. Expression of Rab11a in vivo and in vitro critically depended on c-Fos, because TPA application to the back skin of c-Fos-deficient mice and to mouse embryonic fibroblasts did not induce Rab11a mRNA or protein expression. Moreover, dexamethasone, which is a potent inhibitor of AP-1-mediated transactivation that exhibits anti-inflammatory and anti-tumor promoting activities, inhibited TPA-induced expression of Rab11a. Within the Rab11a gene promoter, we identified a functional AP-1 binding element that exhibited elevated c-Fos binding activity after TPA treatment of keratinocytes. Enhanced expression was not restricted to chemically induced mouse skin tumors but was also found in tumor specimens derived from patients with epithelial skin tumors. These data identify Rab11a as a novel, tumor-associated c-Fos/AP-1 target and may point to an as yet unrecognized function of Rab11a in the development of skin cancer.
Figures
References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Marks F, Fürstenberger G. The conversion stage of skin carcinogenesis. Carcinogenesis. 1990;11:2085–2092. - PubMed
-
- Yuspa SH. The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis. J Dermatol Sci. 1998;17:1–7. - PubMed
-
- Belman S, Troll W. The inhibition of croton oil-promoted mouse skin tumorigenesis by steroid hormones. Cancer Res. 1972;32:450–454. - PubMed
-
- Angel P, Szabowski A, Schorpp-Kistner M. Function and regulation of AP-1 subunits in skin physiology and pathology. Oncogene. 2001;20:2413–2423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

