EGFL7 is a chemoattractant for endothelial cells and is up-regulated in angiogenesis and arterial injury
- PMID: 15972971
- PMCID: PMC1451775
- DOI: 10.1016/S0002-9440(10)62972-0
EGFL7 is a chemoattractant for endothelial cells and is up-regulated in angiogenesis and arterial injury
Abstract
The endothelium of the adult vasculature is normally quiescent, with the exception of the vasculature of the female reproductive system. However, in response to appropriate stimuli (ie, wound healing, atherosclerosis, tumor growth and metastasis, arthritis) the vasculature becomes activated and grows new capillaries through angiogenesis. We have recently identified a novel endothelial-restricted gene, Egfl7, that encodes a 41-kd secreted protein (Fitch MJ, Campagnolo L, Kuhnert F, Stuhlmann H: Egfl7, a novel epidermal growth factor-domain gene expressed in endothelial cells. Dev Dyn 2004, 230:316-324). Egfl7 is expressed at high levels early during mouse embryonic development and is strictly associated with the vascular bed. In this study, we investigated Egfl7 expression in the quiescent adult vasculature, in the pregnant uterus, and in two different models of arterial injury, namely ballooning and ferric chloride injury. By RNA in situ hybridization, Egfl7 expression in the vasculature was found to be restricted to the endothelium of the capillaries and mature vessels. In the pregnant uterus, increased vascularization was accompanied by up-regulation of Egfl7. On arterial injury, Egfl7 expression was up-regulated in the regenerating endothelium, but not in the neointima. Importantly, the EGFL7 protein acted as a chemoattractant for embryonic endothelial cells and fibroblasts in a cell migration assay. Together, these results suggest that Egfl7 functions in the formation and maintenance of endothelial integrity and that its up-regulation may be a critical component in the reorganization of the vascular bed in response to angiogenic stimuli.
Figures

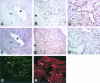
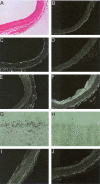


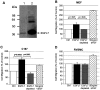
Similar articles
-
EGFL7: a unique angiogenic signaling factor in vascular development and disease.Blood. 2012 Feb 9;119(6):1345-52. doi: 10.1182/blood-2011-10-322446. Epub 2011 Dec 7. Blood. 2012. PMID: 22160377 Free PMC article. Review.
-
EGFL7 is expressed in bone microenvironment and promotes angiogenesis via ERK, STAT3, and integrin signaling cascades.J Cell Physiol. 2015 Jan;230(1):82-94. doi: 10.1002/jcp.24684. J Cell Physiol. 2015. PMID: 24909139
-
Epidermal growth factor-like domain 7 is a marker of the endothelial lineage and active angiogenesis.Genesis. 2014 Jul;52(7):657-70. doi: 10.1002/dvg.22781. Epub 2014 May 2. Genesis. 2014. PMID: 24740971 Free PMC article.
-
Egfl7, a novel epidermal growth factor-domain gene expressed in endothelial cells.Dev Dyn. 2004 Jun;230(2):316-24. doi: 10.1002/dvdy.20063. Dev Dyn. 2004. PMID: 15162510 Free PMC article.
-
Role of the vascular endothelial growth factor isoforms in retinal angiogenesis and DiGeorge syndrome.Verh K Acad Geneeskd Belg. 2005;67(4):229-76. Verh K Acad Geneeskd Belg. 2005. PMID: 16334858 Review.
Cited by
-
CASZ1 promotes vascular assembly and morphogenesis through the direct regulation of an EGFL7/RhoA-mediated pathway.Dev Cell. 2013 Apr 29;25(2):132-43. doi: 10.1016/j.devcel.2013.03.003. Dev Cell. 2013. PMID: 23639441 Free PMC article.
-
EGFL7: a unique angiogenic signaling factor in vascular development and disease.Blood. 2012 Feb 9;119(6):1345-52. doi: 10.1182/blood-2011-10-322446. Epub 2011 Dec 7. Blood. 2012. PMID: 22160377 Free PMC article. Review.
-
Novel role of microRNA-126 in digestive system cancers: From bench to bedside.Oncol Lett. 2019 Jan;17(1):31-41. doi: 10.3892/ol.2018.9639. Epub 2018 Oct 29. Oncol Lett. 2019. PMID: 30655735 Free PMC article. Review.
-
Prostate cancer relevant antigens and enzymes for targeted drug delivery.J Control Release. 2014 Aug 10;187:118-32. doi: 10.1016/j.jconrel.2014.05.035. Epub 2014 May 27. J Control Release. 2014. PMID: 24878184 Free PMC article. Review.
-
Epidermal growth factor-like domain 7 promotes migration and invasion of human trophoblast cells through activation of MAPK, PI3K and NOTCH signaling pathways.Mol Hum Reprod. 2015 May;21(5):435-51. doi: 10.1093/molehr/gav006. Epub 2015 Feb 9. Mol Hum Reprod. 2015. PMID: 25667199 Free PMC article.
References
-
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. - PubMed
-
- Rossant J, Howard L. Signaling pathways in vascular development. Annu Rev Cell Dev Biol. 2002;18:541–573. - PubMed
-
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. - PubMed
-
- Folkman J, D’Amore PA. Bloods vessel formation: what is its molecular basis. Cell. 1996;87:1153–1155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

