Ubiquilin regulates presenilin endoproteolysis and modulates gamma-secretase components, Pen-2 and nicastrin
- PMID: 15975090
- PMCID: PMC1276952
- DOI: 10.1042/BJ20050491
Ubiquilin regulates presenilin endoproteolysis and modulates gamma-secretase components, Pen-2 and nicastrin
Abstract
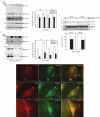
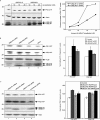
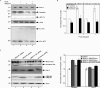
Mutations in presenilin proteins (PS1 and PS2) lead to early-onset Alzheimer's disease. PS proteins are endoproteolytically cleaved into two main fragments: the NTF (PS N-terminal fragment) and the CTF (PS C-terminal fragment). The two fragments are believed to constitute the core catalytic enzyme activity called gamma-secretase, which is responsible for cleaving beta-amyloid precursor protein to release Abeta. Thus, studying factors that modulate PS fragment levels could provide important information about gamma-secretase. Previously, we demonstrated that the protein, ubiquilin-1, interacts both in vivo and in vitro with PS and that overexpression of ubiquilin-1 or -2 leads to increased accumulation of full-length PS proteins. Using wild-type HEK-293 cells (human embryonic kidney 293 cells) and PS-inducible cells, we now show that overexpression of either ubiquilin-1 or -2 decreases the PS NTF and CTF levels. Conversely, siRNA (small interfering RNA)-mediated knockdown of ubiquilin-1 and -2 proteins increased the PS NTF and CTF levels. We considered that ubiquilin might alter PS fragment accumulation by acting as a shuttle factor escorting PS fragments to the proteasome for degradation. However, through proteasome inhibition studies, we show that this does not occur. Instead, our results suggest that ubiquilin regulates PS fragment production. We also examined whether other components of the gamma-secretase complex are affected by ubiquilin expression. Interestingly, overexpression of ubiquilin resulted in a decrease in Pen-2 and nicastrin levels, two essential components of the gamma-secretase complex. In contrast, knockdown of ubiquilin-1 and -2 protein expression by RNAi (RNA interference) increased Pen-2 and nicastrin levels. Finally, we show that inhibition of the proteasome results in decreased PS fragment production and that reversal of proteasome inhibition restores PS fragment production, suggesting that the proteasome may be involved in PS endoproteolysis. These studies implicate ubiquilin as an important factor in regulating PS biogenesis and metabolism.
Figures
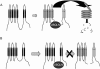


Similar articles
-
gamma-Secretase complexes containing N- and C-terminal fragments of different presenilin origin retain normal gamma-secretase activity.J Neurochem. 2005 Nov;95(3):880-90. doi: 10.1111/j.1471-4159.2005.03415.x. Epub 2005 Aug 31. J Neurochem. 2005. PMID: 16135086
-
PEN-2 enhances gamma-cleavage after presenilin heterodimer formation.J Neurochem. 2004 Sep;90(6):1402-13. doi: 10.1111/j.1471-4159.2004.02597.x. J Neurochem. 2004. PMID: 15341524
-
Co-expressed presenilin 1 NTF and CTF form functional gamma-secretase complexes in cells devoid of full-length protein.J Neurochem. 2004 Apr;89(1):44-53. doi: 10.1046/j.1471-4159.2003.02298.x. J Neurochem. 2004. PMID: 15030388
-
Relationship between presenilinase and gamma-secretase.Drug News Perspect. 2003 Mar;16(2):69-74. doi: 10.1358/dnp.2003.16.2.740248. Drug News Perspect. 2003. PMID: 12792666 Review.
-
Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex.Neuron. 2003 Apr 10;38(1):9-12. doi: 10.1016/s0896-6273(03)00205-8. Neuron. 2003. PMID: 12691659 Review.
Cited by
-
Protein aggregation and degradation mechanisms in neurodegenerative diseases.Am J Neurodegener Dis. 2013;2(1):1-14. Epub 2013 Mar 8. Am J Neurodegener Dis. 2013. PMID: 23516262 Free PMC article.
-
Potentiation of amyotrophic lateral sclerosis (ALS)-associated TDP-43 aggregation by the proteasome-targeting factor, ubiquilin 1.J Biol Chem. 2009 Mar 20;284(12):8083-92. doi: 10.1074/jbc.M808064200. Epub 2008 Dec 26. J Biol Chem. 2009. PMID: 19112176 Free PMC article.
-
APOE*E2 allele delays age of onset in PSEN1 E280A Alzheimer's disease.Mol Psychiatry. 2016 Jul;21(7):916-24. doi: 10.1038/mp.2015.177. Epub 2015 Dec 1. Mol Psychiatry. 2016. PMID: 26619808 Free PMC article.
-
Studies of the role of ubiquitination in the interaction of ubiquilin with the loop and carboxyl terminal regions of presenilin-2.Biochemistry. 2007 Jul 31;46(30):8827-37. doi: 10.1021/bi700604q. Epub 2007 Jul 6. Biochemistry. 2007. PMID: 17614368 Free PMC article.
-
Characterization of the wheat endosperm transfer cell-specific protein TaPR60.Plant Mol Biol. 2009 Sep;71(1-2):81-98. doi: 10.1007/s11103-009-9510-1. Epub 2009 Jun 10. Plant Mol Biol. 2009. PMID: 19513805
References
-
- Edbauer D., Winkler E., Regula J. T., Pesold B., Steiner H., Haass C. Reconstitution of γ-secretase activity. Nat. Cell Biol. 2003;5:486–488. - PubMed
-
- De Strooper B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active γ-secretase complex. Neuron. 2003;38:9–12. - PubMed
-
- Haass C., Steiner H. Alzheimer disease γ-secretase: a complex story of GxGD-type presenilin proteases. Trends Cell Biol. 2002;12:556–562. - PubMed
-
- Takasugi N., Tomita T., Hayashi I., Tsuruoka M., Niimura M., Takahashi Y., Thinakaran G., Iwatsubo T. The role of presenilin cofactors in the γ-secretase complex. Nature (London) 2003;422:438–441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

