Regulation of FAK Ser-722 phosphorylation and kinase activity by GSK3 and PP1 during cell spreading and migration
- PMID: 15975092
- PMCID: PMC1276935
- DOI: 10.1042/BJ20050282
Regulation of FAK Ser-722 phosphorylation and kinase activity by GSK3 and PP1 during cell spreading and migration
Abstract
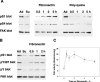
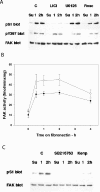
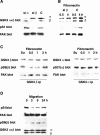
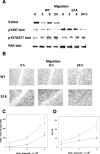
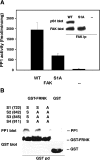
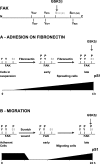
In addition to tyrosine sites, FAK (focal adhesion kinase) is phosphorylated on multiple serine residues. In the present study, the regulation of two of these sites, Ser-722 (S1) and Ser-911 (S4), was investigated. Phosphorylation of S1 (but not S4) decreased in resuspended cells, and recovered during spreading on fibronectin, indicating adhesion-dependent regulation. GSK3 (glycogen synthase kinase 3) inhibitors decreased S1 phosphorylation, and siRNA (short interfering RNA) silencing indicated further the involvement of GSK3beta. Furthermore, GSK3beta was found to become activated during cell spreading on fibronectin, and to physically associate with FAK. S1 phosphorylation was observed to decrease in wounded cell monolayers, while GSK3beta underwent inactivation and later was observed to increase to the original level within 24 h. Direct phosphorylation of S1, requiring pre-phosphorylation of Ser-726 in the +4 position, was demonstrated using purified GSK3 and a synthetic peptide containing FAK residues 714-730. An inhibitory role for S1 phosphorylation in FAK signalling was indicated by findings that both alanine substitution for S1 and dephosphorylation of S1 by PP1 (serine/threonine protein phosphatase type-1) resulted in an increase in FAK kinase activity; likewise, this role was also shown by cell treatment with the GSK3 inhibitor LiCl. The inhibitory role was confirmed by the finding that cells expressing FAK with alanine substitution for S1 displayed improved cell spreading and faster migration in wound-healing and trans-well assays. Finally, the finding that S1 phosphorylation increased in cells treated with the PP1 inhibitor okadaic acid indicated targeting of this site by PP1. These results indicate an additional mechanism for regulation of FAK activity during cell spreading and migration, involving Ser-722 phosphorylation modulated through the competing actions of GSK3beta and PP1.
Figures




Similar articles
-
FAK phosphorylation at Ser-843 inhibits Tyr-397 phosphorylation, cell spreading and migration.J Cell Physiol. 2007 Feb;210(2):436-44. doi: 10.1002/jcp.20870. J Cell Physiol. 2007. PMID: 17096371
-
Cell-cycle-dependent association of protein phosphatase 1 and focal adhesion kinase.Biochem J. 2001 Sep 1;358(Pt 2):407-14. doi: 10.1042/0264-6021:3580407. Biochem J. 2001. PMID: 11513739 Free PMC article.
-
Targeting of FAK Ser910 by ERK5 and PP1delta in non-stimulated and phorbol ester-stimulated cells.Biochem J. 2007 Nov 15;408(1):7-18. doi: 10.1042/BJ20070058. Biochem J. 2007. PMID: 17692050 Free PMC article.
-
FAK-Dependent Cell Motility and Cell Elongation.Cells. 2020 Jan 12;9(1):192. doi: 10.3390/cells9010192. Cells. 2020. PMID: 31940873 Free PMC article. Review.
-
Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases.Pharmacol Ther. 2015 Apr;148:114-31. doi: 10.1016/j.pharmthera.2014.11.016. Epub 2014 Nov 27. Pharmacol Ther. 2015. PMID: 25435019 Free PMC article. Review.
Cited by
-
The role of GSK3beta in the development of the central nervous system.Front Biol (Beijing). 2012 Jun;7(3):212-220. doi: 10.1007/s11515-012-1222-2. Front Biol (Beijing). 2012. PMID: 25688261 Free PMC article.
-
Helicobacter pylori and Epstein-Barr virus infection in cell polarity alterations.Folia Microbiol (Praha). 2024 Feb;69(1):41-57. doi: 10.1007/s12223-023-01091-7. Epub 2023 Sep 6. Folia Microbiol (Praha). 2024. PMID: 37672163 Review.
-
GSK3 as a Sensor Determining Cell Fate in the Brain.Front Mol Neurosci. 2012 Feb 9;5:4. doi: 10.3389/fnmol.2012.00004. eCollection 2012. Front Mol Neurosci. 2012. PMID: 22363258 Free PMC article.
-
Glycogen synthase kinase-3 regulates microglial migration, inflammation, and inflammation-induced neurotoxicity.Cell Signal. 2009 Feb;21(2):264-73. doi: 10.1016/j.cellsig.2008.10.014. Epub 2008 Oct 29. Cell Signal. 2009. PMID: 19007880 Free PMC article.
-
Biological and clinical significance of GSK-3beta in mantle cell lymphoma--an immunohistochemical study.Int J Clin Exp Pathol. 2010 Jan 25;3(3):244-53. Int J Clin Exp Pathol. 2010. PMID: 20224723 Free PMC article.
References
-
- Hanks S. K., Ryzhova L., Shin N., Brabek J. Focal adhesion kinase signalling activities and their implications in the control of cell survival and motility. Front. Biosci. 2003;8:982–986. - PubMed
-
- Parsons J. T. Focal adhesion kinase: the first ten years. J. Cell Sci. 2003;116:1409–1416. - PubMed
-
- Carragher N. O., Frame M. C. Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol. 2004;14:241–249. - PubMed
-
- Owen J. D., Ruest P. J., Fry D. W., Hanks S. K. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol. Cell. Biol. 1999;19:4806–4818. - PMC - PubMed
-
- Malik R. K., Parsons J. T. Integrin-mediated signaling in normal and malignant cells: a role of protein tyrosine kinases. Biochim. Biophys. Acta. 1996;1287:73–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

