Promiscuous interaction of SNAP-25 with all plasma membrane syntaxins in a neuroendocrine cell
- PMID: 15975093
- PMCID: PMC1316263
- DOI: 10.1042/BJ20050583
Promiscuous interaction of SNAP-25 with all plasma membrane syntaxins in a neuroendocrine cell
Abstract
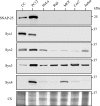
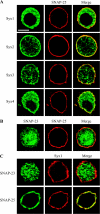
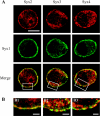
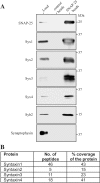
SNAP-25 (25 kDa synaptosome-associated protein) is found in cells that release neurotransmitters and hormones, and plays a central role in the fusion of secretory vesicles with the plasma membrane. SNAP-25 has been shown to interact specifically with syntaxin 1, a 35 kDa membrane protein, to mediate the fusion process. Here, we investigated whether other known syntaxin isoforms found at the plasma membrane can serve as binding partners for SNAP-25 in vivo. In our analysis, we employed rat phaeochromocytoma PC12 cells that are often used as a model of neuronal functions. We now show that these cells contain large amounts of SNAP-25, which interacts not only with syntaxin 1, but also with ubiquitous syntaxins 2, 3 and 4. The plasma membrane syntaxins appear to occupy complementary domains at the plasma membrane. In defined reactions, the ubiquitous plasma membrane syntaxin isoforms, when in binary complexes with SNAP-25, readily bound vesicular synaptobrevin to form SDS-resistant SNARE (soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor) complexes implicated in membrane fusion. However, vesicular synaptotagmin and cytosolic complexin, both implicated in the fusion process, exhibited differential ability to interact with the SNARE complexes formed by syntaxins 1-4, suggesting that the plasma membrane syntaxins may mediate vesicle fusion events with different properties.
Figures


Similar articles
-
SNAP-29 is a promiscuous syntaxin-binding SNARE.Biochem Biophys Res Commun. 2001 Jul 13;285(2):167-71. doi: 10.1006/bbrc.2001.5141. Biochem Biophys Res Commun. 2001. PMID: 11444821
-
A dominant-negative variant of SNAP-23 decreases the cell surface expression of the neuronal glutamate transporter EAAC1 by slowing constitutive delivery.Neurochem Int. 2006 May-Jun;48(6-7):596-603. doi: 10.1016/j.neuint.2005.12.030. Epub 2006 Mar 3. Neurochem Int. 2006. PMID: 16516346
-
Palmitoylation of the 25-kDa synaptosomal protein (SNAP-25) in vitro occurs in the absence of an enzyme, but is stimulated by binding to syntaxin.Biochem J. 2000 Jan 1;345 Pt 1(Pt 1):145-51. Biochem J. 2000. PMID: 10600650 Free PMC article.
-
SNARE complex regulation by phosphorylation.Cell Biochem Biophys. 2006;45(1):111-23. doi: 10.1385/CBB:45:1:111. Cell Biochem Biophys. 2006. PMID: 16679567 Review.
-
The assembly of lipid droplets and its relation to cellular insulin sensitivity.Biochem Soc Trans. 2009 Oct;37(Pt 5):981-5. doi: 10.1042/BST0370981. Biochem Soc Trans. 2009. PMID: 19754436 Review.
Cited by
-
SNARE Proteins in Synaptic Vesicle Fusion.Adv Neurobiol. 2023;33:63-118. doi: 10.1007/978-3-031-34229-5_4. Adv Neurobiol. 2023. PMID: 37615864
-
Syntaxin 3 and SNAP-25 pairing, regulated by omega-3 docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the rod outer segments.J Cell Sci. 2009 Jun 15;122(Pt 12):2003-13. doi: 10.1242/jcs.039982. Epub 2009 May 19. J Cell Sci. 2009. PMID: 19454479 Free PMC article.
-
Murine CENPF interacts with syntaxin 4 in the regulation of vesicular transport.J Cell Sci. 2008 Oct 15;121(Pt 20):3413-21. doi: 10.1242/jcs.032847. Epub 2008 Sep 30. J Cell Sci. 2008. PMID: 18827011 Free PMC article.
-
Mechanism of arachidonic acid action on syntaxin-Munc18.EMBO Rep. 2007 Apr;8(4):414-9. doi: 10.1038/sj.embor.7400935. Epub 2007 Mar 16. EMBO Rep. 2007. PMID: 17363971 Free PMC article.
-
SNAP-25, but not SNAP-23, is essential for photoreceptor development, survival, and function in mice.Commun Biol. 2024 Jan 5;7(1):34. doi: 10.1038/s42003-023-05760-8. Commun Biol. 2024. PMID: 38182732 Free PMC article.
References
-
- Sollner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature (London) 1993;362:318–324. - PubMed
-
- Jahn R., Lang T., Sudhof T. C. Membrane fusion. Cell. 2003;112:519–533. - PubMed
-
- Bock J. B., Matern H. T., Peden A. A., Scheller R. H. A genomic perspective on membrane compartment organization. Nature (London) 2001;409:839–841. - PubMed
-
- Wendler F., Tooze S. Syntaxin 6: the promiscuous behaviour of a SNARE protein. Traffic. 2001;2:606–611. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

