Taxol-stabilized microtubules can position the cytokinetic furrow in mammalian cells
- PMID: 15975912
- PMCID: PMC1196349
- DOI: 10.1091/mbc.e04-11-0974
Taxol-stabilized microtubules can position the cytokinetic furrow in mammalian cells
Abstract
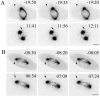
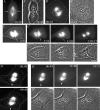
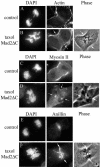
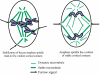
How microtubules act to position the plane of cell division during cytokinesis is a topic of much debate. Recently, we showed that a subpopulation of stable microtubules extends past chromosomes and interacts with the cell cortex at the site of furrowing, suggesting that these stabilized microtubules may stimulate contractility. To test the hypothesis that stable microtubules can position furrows, we used taxol to rapidly suppress microtubule dynamics during various stages of mitosis in PtK1 cells. Cells with stabilized prometaphase or metaphase microtubule arrays were able to initiate furrowing when induced into anaphase by inhibition of the spindle checkpoint. In these cells, few microtubules contacted the cortex. Furrows formed later than usual, were often aberrant, and did not progress to completion. Images showed that furrowing correlated with the presence of one or a few stable spindle microtubule plus ends at the cortex. Actin, myosin II, and anillin were all concentrated in these furrows, demonstrating that components of the contractile ring can be localized by stable microtubules. Inner centromere protein (INCENP) was not found in these ingressions, confirming that INCENP is dispensable for furrow positioning. Taxol-stabilization of the numerous microtubule-cortex interactions after anaphase onset delayed furrow initiation but did not perturb furrow positioning. We conclude that taxol-stabilized microtubules can act to position the furrow and that loss of microtubule dynamics delays the timing of furrow onset and prevents completion. We discuss our findings relative to models for cleavage stimulation.
Figures





Similar articles
-
Induction of cytokinesis is independent of precisely regulated microtubule dynamics.Mol Biol Cell. 2005 Oct;16(10):4485-94. doi: 10.1091/mbc.e05-04-0305. Epub 2005 Jul 12. Mol Biol Cell. 2005. PMID: 16014607 Free PMC article.
-
The Timing of Midzone Stabilization during Cytokinesis Depends on Myosin II Activity and an Interaction between INCENP and Actin.Curr Biol. 2016 Mar 7;26(5):698-706. doi: 10.1016/j.cub.2016.01.018. Epub 2016 Feb 18. Curr Biol. 2016. PMID: 26898472 Free PMC article.
-
Determining the position of the cell division plane.Nature. 2003 Aug 28;424(6952):1074-8. doi: 10.1038/nature01860. Epub 2003 Aug 6. Nature. 2003. PMID: 12904818
-
Centralspindlin in Rappaport's cleavage signaling.Semin Cell Dev Biol. 2016 May;53:45-56. doi: 10.1016/j.semcdb.2016.03.006. Epub 2016 Mar 7. Semin Cell Dev Biol. 2016. PMID: 26964770 Review.
-
Role of microtubules in stimulating cytokinesis in animal cells.Ann N Y Acad Sci. 1990;582:88-98. doi: 10.1111/j.1749-6632.1990.tb21670.x. Ann N Y Acad Sci. 1990. PMID: 1972614 Review.
Cited by
-
Marking and measuring single microtubules by PRC1 and kinesin-4.Cell. 2013 Jul 18;154(2):377-90. doi: 10.1016/j.cell.2013.06.021. Cell. 2013. PMID: 23870126 Free PMC article.
-
Action at a distance during cytokinesis.J Cell Biol. 2009 Dec 14;187(6):831-45. doi: 10.1083/jcb.200907090. J Cell Biol. 2009. PMID: 20008563 Free PMC article.
-
Is inflammatory micronucleation the key to a successful anti-mitotic cancer drug?Open Biol. 2017 Nov;7(11):170182. doi: 10.1098/rsob.170182. Open Biol. 2017. PMID: 29142107 Free PMC article. Review.
-
Phytochemicals as Chemo-Preventive Agents and Signaling Molecule Modulators: Current Role in Cancer Therapeutics and Inflammation.Int J Mol Sci. 2022 Dec 12;23(24):15765. doi: 10.3390/ijms232415765. Int J Mol Sci. 2022. PMID: 36555406 Free PMC article. Review.
-
Synthesis and anticancer activity of all known (-)-agelastatin alkaloids.J Org Chem. 2013 Dec 6;78(23):11970-84. doi: 10.1021/jo4020112. Epub 2013 Nov 21. J Org Chem. 2013. PMID: 24152243 Free PMC article.
References
-
- Adams, R. R., Maiato, H., Earnshaw, W. C., and Carmena, M. (2001). Essential roles of Drosophila inner centromere protein (INCENP) and aurora B. in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J. Cell Biol. 153, 865-880. - PMC - PubMed
-
- Amin-Hanjani, S., and Wadsworth, P. (1991). Inhibition of spindle elongation by taxol. Cell Motil. Cytoskeleton 20, 136-144. - PubMed
-
- Amos, L. A., and Lowe, J. (1999). How Taxol stabilises microtubule structure. Chem. Biol. 6, R65-R69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

