Efficacy and safety of switching from infliximab to adalimumab: a comparative controlled study
- PMID: 15975964
- PMCID: PMC1798023
- DOI: 10.1136/ard.2005.039099
Efficacy and safety of switching from infliximab to adalimumab: a comparative controlled study
Abstract
Objective: To describe the efficacy and safety of adalimumab in patients with rheumatoid arthritis (RA) who had previously discontinued infliximab treatment.
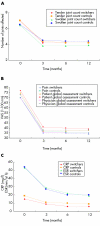
Methods: 24 patients with RA who discontinued treatment with infliximab (switchers) were treated with adalimumab (40 mg every 2 weeks, subcutaneously) for 12 months. The results were compared with those for 25 patients with RA receiving adalimumab who had not previously used an anti-tumour necrosis factor alpha inhibitor (controls). Disease activity was measured with the 28 joint count Disease Activity Score (DAS28), and clinical response with the American College of Rheumatology (ACR) 20% response criteria.
Results: At baseline there were no differences in demographic, clinical, and laboratory features between the two groups. After 12 months' adalimumab treatment, clinical improvement was similar in both groups. More specifically, ACR 20% response criteria were achieved by 18/24 (75%) switchers and by 19/25 (76%) subjects in the control group. Four switchers discontinued the study-two because of adverse events and two because of lack of efficacy, while three control patients discontinued the study-one because of lack of efficacy and two owing to side effects.
Conclusion: Adalimumab is a well tolerated and effective treatment for patients with RA, even when infliximab has been discontinued.
References
-
- Maini R, St Clair E W, Breedveld F, Kalden J, Weisman M, Smolen J.et al Infliximab (chimeric anti‐tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomized phase III trial. ATTRACT Study Group. Lancet 19993541932–1939. - PubMed
-
- Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. - PubMed
-
- Voulgari P V, Alamanos Y, Nikas S N, Bougias D V, Temekonidis T I, Drosos A A. Infliximab therapy in established rheumatoid arthritis: an observational study. Am J Med 2005118515–520. - PubMed
-
- Felson D T, Anderson J J, Boers M, Bombardier C, Furst D, Goldsmith C.et al American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 199538727–735. - PubMed
-
- Paulus H E, Egger M J, Ward J R, Williams H J. Analysis of improvement in individual rheumatoid arthritis patients treated with disease‐modifying antirheumatic drugs, based on the findings in patients treated with placebo. The Cooperative Systematic Studies of Rheumatic Diseases Group. Arthritis Rheum 199033477–484. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical