The effect of etanercept on anti-cyclic citrullinated peptide antibodies and rheumatoid factor in patients with rheumatoid arthritis
- PMID: 15975966
- PMCID: PMC1797988
- DOI: 10.1136/ard.2005.038851
The effect of etanercept on anti-cyclic citrullinated peptide antibodies and rheumatoid factor in patients with rheumatoid arthritis
Abstract
Objective: To evaluate the changes in anti-cyclic citrullinated peptide antibodies (anti-CCP) and rheumatoid factor (RF) following etanercept treatment in patients with rheumatoid arthritis.
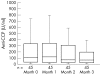
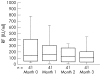
Methods: The study included 90 patients with rheumatoid arthritis who failed treatment with disease modifying antirheumatic drugs (DMARDs). All patients were allowed to continue treatment with DMARDs; 52 of them received etanercept as a twice weekly 25 mg subcutaneous injection for three months, and the others did not. Serum samples were collected at baseline and one month intervals during the treatment course. The serum levels of anti-CCP and RF were tested by enzyme linked immunosorbent assay and nephelometry, respectively.
Results: At baseline, 45 of the 52 etanercept treated patients (86.5%) and 32 of the 38 controls (84.2%) were positive for anti-CCP. Tests for RF were positive in 78.9% and 84.2% of patients with or without etanercept treatment, respectively. The serum levels of anti-CCP and RF decreased significantly after a three month etanercept treatment (p = 0.007 and p = 0.006, respectively). The average decrease from baseline calculated for each individual patient in the etanercept treated group was 31.3% for anti-CCP and 36% for RF. The variation in anti-CCP was positively correlated with the variation in disease activity, swollen and tender joint counts, RF, and C reactive protein.
Conclusions: Etanercept combined with DMARDs leads to a much greater decrease than DMARDs alone in the serum levels of anti-CCP and RF in rheumatoid arthritis, compatible with a reduction in clinical disease activity.
References
-
- Fries J F, Williams C A, Morfeld D, Singh G, Sibley J. Reduction in long‐term disability in patients with rheumatoid arthritis by disease‐modifying antirheumatic drug‐based treatment strategies. Arthritis Rheum 199639616–622. - PubMed
-
- Cash J M, Klippel J H. Second‐line drug therapy for rheumatoid arthritis. N Engl J Med 19943301368–1375. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials