Synergistic interactions between Ca2+ entries through L-type Ca2+ channels and Na+-Ca2+ exchanger in normal and failing rat heart
- PMID: 15975978
- PMCID: PMC1474206
- DOI: 10.1113/jphysiol.2005.091280
Synergistic interactions between Ca2+ entries through L-type Ca2+ channels and Na+-Ca2+ exchanger in normal and failing rat heart
Abstract
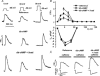
We used confocal Ca2+ imaging and the patch-clamp technique to investigate the interplay between Ca2+ entries through L-type Ca2+ channels (LCCs) and reverse-mode Na+-Ca2+ exchange (NCX) in activating Ca2+-induced Ca2+ release (CICR) from the sarcoplasmic reticulum (SR) in cardiac myocytes from normal and failing rat hearts. In normal myocytes exposed to N(6),2'-O-dibutyryl adenosine-3',5'-cyclic monophosphate (db-cAMP, membrane-permeable form of cAMP), the bell-shaped voltage dependence of cytosolic Ca2+ transients was dramatically broadened due to activation of SR Ca2+ release at high membrane potentials (30-120 mV). This broadening of Ca2+-transient voltage dependence could be prevented by KB-R7943, an inhibitor of the reverse-mode NCX. Trans-sarcolemmal Ca2+ entries were measured fluorometrically in myocytes during depolarizing steps to high membrane potentials. The total Ca2+ entry (deltaF(Tot)) was separated into two Ca2+ entry components, LCC-mediated (deltaF(LCC)) and NCX-mediated (deltaF(NCX)), by exposing the cells to the specific inhibitors of LCCs and reverse-mode NCX, nifedipine and KB-R7943, respectively. In the absence of protein kinase A (PKA) stimulation the amplitude of the Ca2+-inflow signal (deltaF(Tot)) corresponded to the arithmetic sum of the amplitudes of the KB-R7943- and nifedipine-resistant components (deltaF(Tot)=deltaF(LCC)+deltaF(NCX)). PKA activation resulted in significant increases in deltaF(Tot) and deltaF(LCC). Paradoxically, deltaF(Tot) became approximately threefold larger than the sum of the deltaF(NCX) and deltaF(LCC) components. In myocytes from failing hearts, stimulation of PKA failed to induce a shift in Ca2+ release voltage dependence toward more positive membrane potentials. Although the total and NCX-mediated Ca2+ entries were increased again, deltaF(Tot) did not significantly exceed the sum of deltaF(LCC) and deltaF(NCX). We conclude that the LCC and NCX Ca2+-entry pathways interact synergistically to trigger SR Ca2+ release on depolarization to positive membrane potentials in PKA-stimulated cardiac muscle. In heart failure, this new form of Ca2+ release is diminished and may potentially account for the compromised contractile performance and reduced functional reserve in failing hearts.
Figures
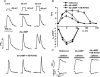




Similar articles
-
Intracellular [Na(+)] modulates synergy between Na(+)/Ca (2+) exchanger and L-type Ca (2+) current in cardiac excitation-contraction coupling during action potentials.Basic Res Cardiol. 2011 Nov;106(6):967-77. doi: 10.1007/s00395-011-0202-z. Epub 2011 Jul 21. Basic Res Cardiol. 2011. PMID: 21779914
-
Ontogeny of Ca2+-induced Ca2+ release in rabbit ventricular myocytes.Am J Physiol Cell Physiol. 2008 Feb;294(2):C516-25. doi: 10.1152/ajpcell.00417.2007. Epub 2007 Dec 19. Am J Physiol Cell Physiol. 2008. PMID: 18094144
-
Calcium entry via Na/Ca exchange during the action potential directly contributes to contraction of failing human ventricular myocytes.Cardiovasc Res. 2003 Mar 15;57(4):974-85. doi: 10.1016/s0008-6363(02)00732-0. Cardiovasc Res. 2003. PMID: 12650875
-
Cardiac myocytes Ca2+ and Na+ regulation in normal and failing hearts.J Pharmacol Sci. 2006;100(5):315-22. doi: 10.1254/jphs.cpj06001x. Epub 2006 Mar 18. J Pharmacol Sci. 2006. PMID: 16552170 Review.
-
Action potential duration modulates calcium influx, Na(+)-Ca2+ exchange, and intracellular calcium release in rat ventricular myocytes.Ann N Y Acad Sci. 1996 Apr 15;779:417-29. doi: 10.1111/j.1749-6632.1996.tb44817.x. Ann N Y Acad Sci. 1996. PMID: 8659858 Review.
Cited by
-
Contribution of the Na+/Ca2+ exchanger to rapid Ca2+ release in cardiomyocytes.Biophys J. 2006 Aug 1;91(3):779-92. doi: 10.1529/biophysj.105.072447. Epub 2006 May 5. Biophys J. 2006. PMID: 16679359 Free PMC article.
-
Modeling Na+-Ca2+ exchange in the heart: Allosteric activation, spatial localization, sparks and excitation-contraction coupling.J Mol Cell Cardiol. 2016 Oct;99:174-187. doi: 10.1016/j.yjmcc.2016.06.068. Epub 2016 Jul 2. J Mol Cell Cardiol. 2016. PMID: 27377851 Free PMC article.
-
Neuronal sodium channels: emerging components of the nano-machinery of cardiac calcium cycling.J Physiol. 2017 Jun 15;595(12):3823-3834. doi: 10.1113/JP273058. Epub 2017 Mar 26. J Physiol. 2017. PMID: 28195313 Free PMC article. Review.
-
Function and regulation of the Na+-Ca2+ exchanger NCX3 splice variants in brain and skeletal muscle.J Biol Chem. 2014 Apr 18;289(16):11293-11303. doi: 10.1074/jbc.M113.529388. Epub 2014 Mar 10. J Biol Chem. 2014. PMID: 24616101 Free PMC article.
-
Cardiac Na+-Ca2+ exchanger: dynamics of Ca2+-dependent activation and deactivation in intact myocytes.J Physiol. 2013 Apr 15;591(8):2067-86. doi: 10.1113/jphysiol.2013.252080. Epub 2013 Feb 11. J Physiol. 2013. PMID: 23401616 Free PMC article.
References
-
- Balijepalli RC, Lokuta AJ, Maertz NA, Buck JM, Haworth RA, Valdivia HH, Kamp TJ. Depletion of T-tubules and specific subcellular changes in sarcolemmal proteins in tachycardia-induced heart failure. Cardiovasc Res. 2003;59:67–77. - PubMed
-
- Bers DM. Excitation–Contraction Coupling and Cardiac Contractile Force. Dordrecht, The Netherlands: Kluwer; 2001.
-
- Bers DM, Eisner DA, Valdivia HH. Sarcoplasmic reticulum Ca2+ and heart failure: roles of diastolic leak and Ca2+ transport. Circ Res. 2003;93:487–490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

