Endothermic force generation, temperature-jump experiments and effects of increased [MgADP] in rabbit psoas muscle fibres
- PMID: 15975981
- PMCID: PMC1474189
- DOI: 10.1113/jphysiol.2005.090084
Endothermic force generation, temperature-jump experiments and effects of increased [MgADP] in rabbit psoas muscle fibres
Abstract
We studied, by experiment and by kinetic modelling, the characteristics of the force increase on heating (endothermic force) in muscle. Experiments were done on maximally Ca2+-activated, permeabilized, single fibres (length approximately 2 mm; sarcomere length, 2.5 microm) from rabbit psoas muscle; [MgATP] was 4.6 mM, pH 7.1 and ionic strength was 200 mM. A small-amplitude (approximately 3 degrees C) rapid laser temperature-jump (0.2 ms T-jump) at 8-9 degrees C induced a tension rise to a new steady state and it consisted of two (fast and slow) exponential components. The T-jump-induced tension rise became slower as [MgADP] was increased, with half-maximal effect at 0.5 mM [MgADP]; the pre- and post-T-jump tension increased approximately 20% with 4 mM added [MgADP]. As determined by the tension change to small, rapid length steps (<1.4%L0 complete in <0.5 ms), the increase of force by [MgADP] was not associated with a concomitant increase of stiffness; the quick tension recovery after length steps (Huxley-Simmons phase 2) was slower with added MgADP. In steady-state experiments, the tension was larger at higher temperatures and the plot of tension versus reciprocal absolute temperature was sigmoidal, with a half-maximal tension at 10-12 degrees C; the relation with added 4 mM MgADP was shifted upwards on the tension axis and towards lower temperatures. The potentiation of tension with 4 mM added MgADP was 20-25% at low temperatures (approximately 5-10 degrees C), but approximately 10% at the physiological temperatures (approximately 30 degrees C). The shortening velocity was decreased with increased [MgADP] at low and high temperatures. The sigmoidal relation between tension and reciprocal temperature, and the basic effects of increased [MgADP] on endothermic force, can be qualitatively simulated using a five-step kinetic scheme for the crossbridge/A-MATPase cycle where the force generating conformational change occurs in a reversible step before the release of inorganic phosphate (P(i)), it is temperature sensitive (Q10 of approximately 4) and the release of MgADP occurs by a subsequent, slower, two-step mechanism. Modelling shows that the sigmoidal relation between force and reciprocal temperature arises from conversion of preforce-generating (A-M.ADP.P(i)) states to force-bearing (A-M.ADP) states as the temperature is raised. A tension response to a simulated T-jump consists of three (one fast and two slow) components, but, by combining the two slow components, they could be reduced to two; their relative amplitudes vary with temperature. The model can qualitatively simulate features of the tension responses induced by large-T-jumps from low starting temperatures, and those induced by small-T-jumps from different starting temperatures and, also, the interactive effects of P(i) and temperature on force in muscle fibres.
Figures


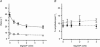
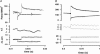
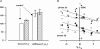
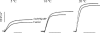
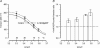
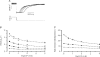




References
-
- Bershitsky SY, Tsaturyan AK, Bershitskaya ON, Mashanov P, Brown P, Burns B, Ferenczi MA. Muscle force is generated by myosin heads stereo-specifically attached to actin. Nature. 1997;388:186–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

