Fetal cardiovascular, metabolic and endocrine responses to acute hypoxaemia during and following maternal treatment with dexamethasone in sheep
- PMID: 15975982
- PMCID: PMC1474208
- DOI: 10.1113/jphysiol.2005.089805
Fetal cardiovascular, metabolic and endocrine responses to acute hypoxaemia during and following maternal treatment with dexamethasone in sheep
Abstract
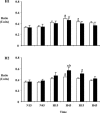
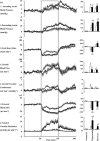
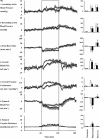
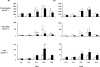
In sheep, direct fetal treatment with dexamethasone alters basal cardiovascular function and the cardiovascular response to acute hypoxaemia. However, in human clinical practice, dexamethasone is administered to the mother, not to the fetus. Hence, this study investigated physiological responses to acute hypoxaemia in fetal sheep during and following maternal treatment with dexamethasone in doses and at dose intervals used in human clinical practice. Under anaesthesia, 18 fetal sheep were instrumented with vascular and amniotic catheters, a carotid flow probe and a femoral flow probe at 118 days gestation (term ca 145 days). Following 6 days recovery at 124 days gestation, 10 ewes received dexamethasone (2 x 12 mg daily i.m. injections in saline). The remaining animals were saline-injected as age-matched controls. Two episodes of hypoxaemia (H) were induced in all animals by reducing the maternal F(IO2)for 1 h (H1, 8 h after the second injection; H2, 3 days after the second injection). In fetuses whose mothers received saline, hypoxaemia induced significant increases in fetal arterial blood pressure, carotid blood flow and carotid vascular conductance and femoral vascular resistance, significant falls in femoral blood flow and femoral vascular conductance and transient bradycardia. These cardiovascular responses were accompanied by a fall in arterial pH, increases in blood glucose and blood lactate concentrations and increased plasma concentrations of catecholamines. In fetuses whose mothers were treated with dexamethasone, bradycardia persisted throughout hypoxaemia, the magnitude of the femoral vasoconstriction, the glycaemic, lactacidaemic and acidaemic responses and the plasma concentration of neuropeptide Y (NPY) were all enhanced during H1. However, during H2, all of these physiological responses were similar to saline controls. In dexamethasone fetuses, the increase in plasma adrenaline was attenuated during H1 and the increase in carotid vascular conductance during hypoxaemia failed to reach statistical significance both during H1 and during H2. These data show that maternal treatment with dexamethasone in doses and intervals used in human obstetric practice modified the fetal cardiovascular, metabolic and endocrine defence responses to acute hypoxaemia. Furthermore, dexamethasone-induced alterations to these defences depended on whether the hypoxaemic challenge occurred during or following maternal dexamethasone treatment.
Figures
Similar articles
-
Cardiovascular and endocrine responses to acute hypoxaemia during and following dexamethasone infusion in the ovine fetus.J Physiol. 2003 May 15;549(Pt 1):271-87. doi: 10.1113/jphysiol.2002.036418. Epub 2003 Mar 28. J Physiol. 2003. PMID: 12665612 Free PMC article.
-
Effects of prevailing hypoxaemia, acidaemia or hypoglycaemia upon the cardiovascular, endocrine and metabolic responses to acute hypoxaemia in the ovine fetus.J Physiol. 2002 Apr 1;540(Pt 1):351-66. doi: 10.1113/jphysiol.2001.013434. J Physiol. 2002. PMID: 11927692 Free PMC article.
-
Adrenocortical responsiveness is blunted in twin relative to singleton ovine fetuses.J Physiol. 2004 Jun 15;557(Pt 3):1021-32. doi: 10.1113/jphysiol.2004.061796. Epub 2004 Apr 8. J Physiol. 2004. PMID: 15073282 Free PMC article.
-
The fetal llama versus the fetal sheep: different strategies to withstand hypoxia.High Alt Med Biol. 2003 Summer;4(2):193-202. doi: 10.1089/152702903322022794. High Alt Med Biol. 2003. PMID: 12855051 Review.
-
Fetal metabolic responses to hypoxia.Reprod Fertil Dev. 1995;7(3):527-38. doi: 10.1071/rd9950527. Reprod Fertil Dev. 1995. PMID: 8606965 Review.
Cited by
-
Asphyxia and therapeutic hypothermia modulate plasma nitrite concentrations and carotid vascular resistance in preterm fetal sheep.Reprod Sci. 2014 Dec;21(12):1483-91. doi: 10.1177/1933719114530187. Epub 2014 Apr 16. Reprod Sci. 2014. PMID: 24740991 Free PMC article.
-
Judicious use of antenatal glucocorticoids: putting the risks into the balance.Facts Views Vis Obgyn. 2011;3(3):215-20. Facts Views Vis Obgyn. 2011. PMID: 24753867 Free PMC article.
-
Magnesium sulphate and cardiovascular and cerebrovascular adaptations to asphyxia in preterm fetal sheep.J Physiol. 2016 Mar 1;594(5):1281-93. doi: 10.1113/JP270614. Epub 2015 Jul 8. J Physiol. 2016. PMID: 26077461 Free PMC article.
-
Metabolic Consequences of Glucocorticoid Exposure before Birth.Nutrients. 2022 May 30;14(11):2304. doi: 10.3390/nu14112304. Nutrients. 2022. PMID: 35684104 Free PMC article. Review.
-
Altered Cardiovascular Defense to Hypotensive Stress in the Chronically Hypoxic Fetus.Hypertension. 2020 Oct;76(4):1195-1207. doi: 10.1161/HYPERTENSIONAHA.120.15384. Epub 2020 Aug 31. Hypertension. 2020. PMID: 32862711 Free PMC article.
References
-
- Anwar MA, Schwab M, Poston L, Nathanielsz PW. Betamethasone-mediated vascular dysfunction and changes in hematological profile in the ovine fetus. Am J Physiol. 1999;276:H1137–H1143. - PubMed
-
- Balasubramaniam A. Neuropeptide Y (NPY) family of hormones: progress in the development of receptor selective agonists and antagonists. Curr Pharm Des. 2003;9:1165–1175. - PubMed
-
- Bartelds B, van Bel F, Teitel DF, Rudolph AM. Carotid, not aortic, chemoreceptors mediate the fetal cardiovascular response to acute hypoxemia in lambs. Pediatr Res. 1993;34:51–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

