Fasciclin II signals new synapse formation through amyloid precursor protein and the scaffolding protein dX11/Mint
- PMID: 15976083
- PMCID: PMC6724788
- DOI: 10.1523/JNEUROSCI.1144-05.2005
Fasciclin II signals new synapse formation through amyloid precursor protein and the scaffolding protein dX11/Mint
Abstract
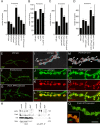
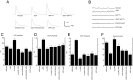
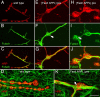
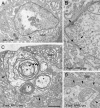
Cell adhesion molecules (CAMs) have been universally recognized for their essential roles during synapse remodeling. However, the downstream pathways activated by CAMs have remained mostly unknown. Here, we used the Drosophila larval neuromuscular junction to investigate the pathways activated by Fasciclin II (FasII), a transmembrane CAM of the Ig superfamily, during synapse remodeling. We show that the ability of FasII to stimulate or to prevent synapse formation depends on the symmetry of transmembrane FasII levels in the presynaptic and postsynaptic cell and requires the presence of the fly homolog of amyloid precursor protein (APPL). In turn, APPL is regulated by direct interactions with the PDZ (postsynaptic density-95/Discs large/zona occludens-1)-containing protein dX11/Mint/Lin-10, which also regulates synapse expansion downstream of FasII. These results provide a novel mechanism by which cell adhesion molecules are regulated and provide fresh insights into the normal operation of APP during synapse development.
Figures



References
-
- Ando K, Iijima KI, Elliott JI, Kirino Y, Suzuki T (2001) Phosphorylation-dependent regulation of the interaction of amyloid precursor protein with Fe65 affects the production of beta-amyloid. J Biol Chem 276: 40353-40361. - PubMed
-
- Aplin AE, Gibb GM, Jacobsen JS, Gallo JM, Anderton BH (1996) In vitro phosphorylation of the cytoplasmic domain of the amyloid precursor protein by glycogen synthase kinase-3beta. J Neurochem 67: 699-707. - PubMed
-
- Bailey CH, Chen M, Keller F, Kandel ER (1992) Serotonin-mediated endocytosis of apCAM: an early step of learning-related synaptic growth in Aplysia Science 256: 645-649. - PubMed
-
- Bailey CH, Kaang BK, Chen M, Martin KC, Lim CS, Casadio A, Kandel ER (1997) Mutation in the phosphorylation sites of MAP kinase blocks learning-related internalization of apCAM in Aplysia sensory neurons. Neuron 18: 913-924. - PubMed
-
- Beggs HE, Baragona SC, Hemperly JJ, Maness PF (1997) NCAM140 interacts with the focal adhesion kinase p125(fak) and the SRC-related tyrosine kinase p59(fyn). J Biol Chem 272: 8310-8319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
