Long-term potentiation is impaired in middle-aged rats: regional specificity and reversal by adenosine receptor antagonists
- PMID: 15976084
- PMCID: PMC6724797
- DOI: 10.1523/JNEUROSCI.0880-05.2005
Long-term potentiation is impaired in middle-aged rats: regional specificity and reversal by adenosine receptor antagonists
Abstract
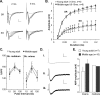
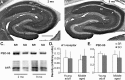
Memory loss in humans begins early in adult life and progresses thereafter. It is not known whether these losses reflect the failure of cellular processes that encode memory or disturbances in events that retrieve it. Here, we report that impairments in hippocampal long-term potentiation (LTP), a form of synaptic plasticity associated with memory, are present by middle age in rats but only in select portions of pyramidal cell dendritic trees. Specifically, LTP induced with theta-burst stimulation in basal dendrites of hippocampal field CA1 decayed rapidly in slices prepared from 7- to 10-month-old rats but not in slices from young adults. There were no evident age-related differences in LTP in the apical dendrites. Both the adenosine A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine and a positive AMPA receptor modulator (ampakine) offset age-related LTP deficits. Adenosine produced greater depression of synaptic responses in middle-aged versus young adult slices and in basal versus apical dendrites. These results were not associated with variations in A1 receptor densities and may instead reflect regional and age-related differences in adenosine clearance. Pertinent to this, brief applications of A1 receptor antagonists immediately after theta stimulation fully restored LTP in middle-aged rats. We hypothesize that the build-up of extracellular adenosine during theta activity persists into the postinduction period in the basal dendrites of middle-aged slices and thereby activates the A1 receptor-dependent LTP reversal effect. Regardless of the underlying mechanism, the present results provide a candidate explanation for memory losses during normal aging and indicate that, with regard to plasticity, different segments of pyramidal neurons age at different rates.
Figures






References
-
- Abraham WC, Huggett A (1997) Induction and reversal of long-term potentiation by repeated high-frequency stimulation in rat hippocampal slices. Hippocampus 7: 137-145. - PubMed
-
- Arai A, Lynch G (1992) Factors regulating the magnitude of long-term potentiation induced by theta pattern stimulation. Brain Res 598: 173-184. - PubMed
-
- Arai A, Larson J, Lynch G (1990a) Anoxia reveals a vulnerable period in the development of long-term potentiation. Brain Res 511: 353-357. - PubMed
-
- Arai A, Kessler M, Lynch G (1990b) The effects of adenosine on the development of long-term potentiation. Neurosci Lett 119: 41-44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
