Intravesicular localization and exocytosis of alpha-synuclein and its aggregates
- PMID: 15976091
- PMCID: PMC6724798
- DOI: 10.1523/JNEUROSCI.0692-05.2005
Intravesicular localization and exocytosis of alpha-synuclein and its aggregates
Abstract
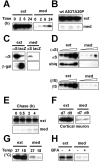
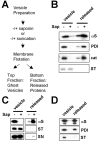
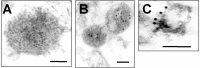
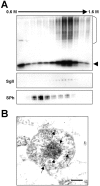
Alpha-synuclein (alpha-syn), particularly in its aggregated forms, is implicated in the pathogenesis of Parkinson's disease and other related neurological disorders. However, the normal biology of alpha-syn and how it relates to the aggregation of the protein are not clearly understood. Because of the lack of the signal sequence and its predominant localization in the cytosol, alpha-syn is generally considered exclusively an intracellular protein. Contrary to this assumption, here, we show that a small percentage of newly synthesized alpha-syn is rapidly secreted from cells via unconventional, endoplasmic reticulum/Golgi-independent exocytosis. Consistent with this finding, we also demonstrate that a portion of cellular alpha-syn is present in the lumen of vesicles. Importantly, the intravesicular alpha-syn is more prone to aggregation than the cytosolic protein, and aggregated forms of alpha-syn are also secreted from cells. Furthermore, secretion of both monomeric and aggregated alpha-syn is elevated in response to proteasomal and mitochondrial dysfunction, cellular defects that are associated with Parkinson's pathogenesis. Thus, intravesicular localization and secretion are part of normal life cycle of alpha-syn and might also contribute to pathological function of this protein.
Figures


References
-
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A (2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25: 239-252. - PubMed
-
- Borghi R, Marchese R, Negro A, Marinelli L, Forloni G, Zaccheo D, Abbruzzese G, Tabaton M (2000) Full length alpha-synuclein is present in cerebrospinal fluid from Parkinson's disease and normal subjects. Neurosci Lett 287: 65-67. - PubMed
-
- Bussell Jr R, Eliezer D (2003) A structural and functional role for 11-mer repeats in alpha-synuclein and other exchangeable lipid binding proteins. J Mol Biol 329: 763-778. - PubMed
-
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL (2002) Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking α-synuclein. J Neurosci 22: 8797-8807. - PMC - PubMed
-
- Chandra S, Chen X, Rizo J, Jahn R, Sudhof TC (2003) A broken alpha-helix in folded alpha-synuclein. J Biol Chem 278: 15313-15318. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
