Elementary response of olfactory receptor neurons to odorants
- PMID: 15976304
- PMCID: PMC2957801
- DOI: 10.1126/science.1109886
Elementary response of olfactory receptor neurons to odorants
Abstract
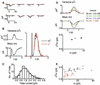
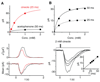
Signaling by heterotrimeric GTP-binding proteins (G proteins) drives numerous cellular processes. The number of G protein molecules activated by a single membrane receptor is a determinant of signal amplification, although in most cases this parameter remains unknown. In retinal rod photoreceptors, a long-lived photoisomerized rhodopsin molecule activates many G protein molecules (transducins), yielding substantial amplification and a large elementary (single-photon) response, before rhodopsin activity is terminated. Here we report that the elementary response in olfactory transduction is extremely small. A ligand-bound odorant receptor has a low probability of activating even one G protein molecule because the odorant dwell-time is very brief. Thus, signal amplification in olfactory transduction appears fundamentally different from that of phototransduction.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

