Immunocytochemical detection of deoxycytidine kinase in haematological malignancies and solid tumours
- PMID: 15976334
- PMCID: PMC1770710
- DOI: 10.1136/jcp.2004.023861
Immunocytochemical detection of deoxycytidine kinase in haematological malignancies and solid tumours
Abstract
Background: Deoxycytidine kinase (dCK) is responsible for the activation of several clinically important deoxynucleoside analogues used for the treatment of haematological and solid malignancies.
Aim: To measure dCK expression in tumour cells from different origins.
Method: A rabbit antihuman dCK antibody was used for the immunocytochemical detection of dCK expression in three leukaemic cell lines (HL60, U937, and CCRF-CEM) and 97 patient samples (paediatric acute myeloid leukaemia (AML) and lymphoid leukaemia (ALL), retinoblastoma, paediatric brain tumours, and adult non-small cell lung cancer (NSCLC)).
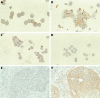
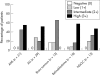
Results: CCRF-CEM, U937, and HL60 cells stained positively for dCK and the degree of expression correlated with dCK activity. dCK expression varied between tumour types and between individual patients within one tumour type. dCK was located predominantly in the cytoplasm. The staining intensity was scored as negative (0), low (1+), intermediate (2+), or high (3+). Expression of dCK was high in AML blasts. In contrast, brain tumour samples expressed low amounts of dCK. dCK staining ranged from low (1+) to high (3+) in ALL blasts, retinoblastoma, and NSCLC tissue samples. Staining was consistent (interobserver variability, 88%; kappa = 0.83) and specific. Western blotting detected the dCK protein appropriately at 30 kDa, without additional bands.
Conclusions: Immunocytochemistry is an effective and reliable method for determining the expression of dCK in patient samples and requires little tumour material. This method enables large scale screening of dCK expression in tumour samples.
Figures

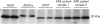
Similar articles
-
Retroviral transfer of deoxycytidine kinase into tumor cell lines enhances nucleoside toxicity.Cancer Res. 1996 May 15;56(10):2343-7. Cancer Res. 1996. PMID: 8625309
-
Activation of deoxycytidine kinase by protein kinase inhibitors and okadaic acid in leukemic cells.Biochem Pharmacol. 2004 Jul 1;68(1):95-103. doi: 10.1016/j.bcp.2004.02.031. Biochem Pharmacol. 2004. PMID: 15183121
-
Expression of deoxycytidine kinase in leukaemic cells compared with solid tumour cell lines, liver metastases and normal liver.Eur J Cancer. 2003 Mar;39(5):691-7. doi: 10.1016/s0959-8049(02)00813-4. Eur J Cancer. 2003. PMID: 12628850
-
Up-regulation of proline-rich tyrosine kinase 2 in non-small cell lung cancer.Lung Cancer. 2008 Dec;62(3):295-301. doi: 10.1016/j.lungcan.2008.05.008. Epub 2008 Jun 20. Lung Cancer. 2008. PMID: 18571765
-
Properties and levels of deoxynucleoside kinases in normal and tumor cells; implications for chemotherapy.Adv Enzyme Regul. 1994;34:13-25. doi: 10.1016/0065-2571(94)90006-x. Adv Enzyme Regul. 1994. PMID: 7942271 Review.
Cited by
-
Molecular Imaging of Deoxycytidine Kinase Activity Using Deoxycytidine-Enhanced CEST MRI.Cancer Res. 2019 May 15;79(10):2775-2783. doi: 10.1158/0008-5472.CAN-18-3565. Epub 2019 Apr 2. Cancer Res. 2019. PMID: 30940660 Free PMC article.
-
dCK Expression and Gene Polymorphism With Gemcitabine Chemosensitivity in Patients With Pancreatic Ductal Adenocarcinoma: A Strobe-Compliant Observational Study.Medicine (Baltimore). 2016 Mar;95(10):e2936. doi: 10.1097/MD.0000000000002936. Medicine (Baltimore). 2016. PMID: 26962792 Free PMC article.
-
Genetic factors influencing cytarabine therapy.Pharmacogenomics. 2009 Oct;10(10):1657-74. doi: 10.2217/pgs.09.118. Pharmacogenomics. 2009. PMID: 19842938 Free PMC article. Review.
-
New approaches to pharmacotherapy of tumors of the nervous system during childhood and adolescence.Pharmacol Ther. 2009 Apr;122(1):44-55. doi: 10.1016/j.pharmthera.2009.01.001. Epub 2009 Jan 23. Pharmacol Ther. 2009. PMID: 19318043 Free PMC article. Review.
-
Cloning and expression pattern of alkaline phosphatase during the development of Paralichthys olivaceus.Fish Physiol Biochem. 2011 Sep;37(3):411-24. doi: 10.1007/s10695-010-9441-4. Epub 2010 Oct 5. Fish Physiol Biochem. 2011. PMID: 20922565
References
-
- Bohman C, Eriksson S. Deoxycytidine kinase from human leukemic spleen: preparation and characteristics of homogeneous enzyme. Biochemistry 1988;27:4258–65. - PubMed
-
- Arner ES, Eriksson S. Mammalian deoxyribonucleoside kinases. Pharmacol Ther 1995;67:155–86. - PubMed
-
- De Clercq E. Antiviral drugs: current state of the art. J Clin Virol 2001;22:73–89. - PubMed
-
- Peters GJ, Van der Wilt CL, Van Moorsel CJ, et al. Basis for effective combination cancer chemotherapy with antimetabolites. Pharmacol Ther 2000;87:227–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
