Co-ordinated bacteriocin production and competence development: a possible mechanism for taking up DNA from neighbouring species
- PMID: 15978073
- PMCID: PMC1262684
- DOI: 10.1111/j.1365-2958.2005.04695.x
Co-ordinated bacteriocin production and competence development: a possible mechanism for taking up DNA from neighbouring species
Abstract
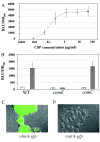
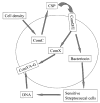
It is important to ensure DNA availability when bacterial cells develop competence. Previous studies in Streptococcus pneumoniae demonstrated that the competence-stimulating peptide (CSP) induced autolysin production and cell lysis of its own non-competent cells, suggesting a possible active mechanism to secure a homologous DNA pool for uptake and recombination. In this study, we found that in Streptococcus mutans CSP induced co-ordinated expression of competence and mutacin production genes. This mutacin (mutacin IV) is a non-lantibiotic bacteriocin which kills closely related Streptococcal species such as S. gordonii. In mixed cultures of S. mutans and S. gordonii harbouring a shuttle plasmid, plasmid DNA transfer from S. gordonii to S. mutans was observed in a CSP and mutacin IV-dependent manner. Further analysis demonstrated an increased DNA release from S. gordonii upon addition of the partially purified mutacin IV extract. On the basis of these findings, we propose that Streptococcus mutans, which resides in a multispecies oral biofilm, may utilize the competence-induced bacteriocin production to acquire transforming DNA from other species living in the same ecological niche. This hypothesis is also consistent with a well-known phenomenon that a large genomic diversity exists among different S. mutans strains. This diversity may have resulted from extensive horizontal gene transfer.
Figures


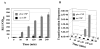
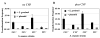

Similar articles
-
Interactions between oral bacteria: inhibition of Streptococcus mutans bacteriocin production by Streptococcus gordonii.Appl Environ Microbiol. 2005 Jan;71(1):354-62. doi: 10.1128/AEM.71.1.354-362.2005. Appl Environ Microbiol. 2005. PMID: 15640209 Free PMC article.
-
Cell density- and ComE-dependent expression of a group of mutacin and mutacin-like genes in Streptococcus mutans.FEMS Microbiol Lett. 2006 Dec;265(1):11-7. doi: 10.1111/j.1574-6968.2006.00459.x. Epub 2006 Sep 19. FEMS Microbiol Lett. 2006. PMID: 16981904
-
LuxS controls bacteriocin production in Streptococcus mutans through a novel regulatory component.Mol Microbiol. 2005 Aug;57(4):960-9. doi: 10.1111/j.1365-2958.2005.04733.x. Mol Microbiol. 2005. PMID: 16091037
-
The mutacins of Streptococcus mutans: regulation and ecology.Mol Oral Microbiol. 2012 Apr;27(2):57-69. doi: 10.1111/j.2041-1014.2011.00634.x. Epub 2011 Dec 23. Mol Oral Microbiol. 2012. PMID: 22394465 Free PMC article. Review.
-
Competence-induced fratricide in streptococci.Mol Microbiol. 2007 Jun;64(6):1423-33. doi: 10.1111/j.1365-2958.2007.05757.x. Mol Microbiol. 2007. PMID: 17555432 Review.
Cited by
-
Quorum Sensing in Streptococcus mutans Regulates Production of Tryglysin, a Novel RaS-RiPP Antimicrobial Compound.mBio. 2021 Mar 16;12(2):e02688-20. doi: 10.1128/mBio.02688-20. mBio. 2021. PMID: 33727351 Free PMC article.
-
Genetic variability of mutans streptococci revealed by wide whole-genome sequencing.BMC Genomics. 2013 Jun 28;14:430. doi: 10.1186/1471-2164-14-430. BMC Genomics. 2013. PMID: 23805886 Free PMC article.
-
Amino Sugars Reshape Interactions between Streptococcus mutans and Streptococcus gordonii.Appl Environ Microbiol. 2020 Dec 17;87(1):e01459-20. doi: 10.1128/AEM.01459-20. Print 2020 Dec 17. Appl Environ Microbiol. 2020. PMID: 33097515 Free PMC article.
-
Core-gene-encoded peptide regulating virulence-associated traits in Streptococcus mutans.J Bacteriol. 2013 Jun;195(12):2912-20. doi: 10.1128/JB.00189-13. Epub 2013 Apr 19. J Bacteriol. 2013. PMID: 23603743 Free PMC article.
-
Competence-dependent endogenous DNA rearrangement and uptake of extracellular DNA give a natural variant of Streptococcus mutans without biofilm formation.J Bacteriol. 2011 Oct;193(19):5147-54. doi: 10.1128/JB.05240-11. Epub 2011 Jul 29. J Bacteriol. 2011. PMID: 21804005 Free PMC article.
References
-
- Aspiras MB, Ellen RP, Cvitkovitch DG. ComX activity of Streptococcus mutans growing in biofilms. FEMS Microbiol Lett. 2004;238:167–174. - PubMed
-
- Baba T, Schneewind O. Instruments of microbial warfare: bacteriocin synthesis, toxicity and immunity. Trends Microbiol. 1998;6:66–71. - PubMed
-
- Baca P, Liebana J, Piedrola G. Epidemiological application of a new bacteriocin typing scheme for Streptococcus mutans. Community Dent Oral Epidemiol. 1990;18:194–196. - PubMed
-
- Bondi M, Neglia RG, Messi P, Manicardi G, Fabio U. Streptococcus mutans: classification in bacteriocin-types. Microbiologica. 1991;14:223–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

