Caveolin-3 is adjacent to a group of extradyadic ryanodine receptors
- PMID: 15980179
- PMCID: PMC1366692
- DOI: 10.1529/biophysj.105.064212
Caveolin-3 is adjacent to a group of extradyadic ryanodine receptors
Abstract
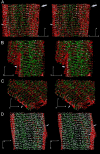
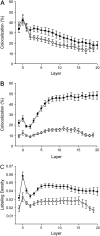
Caveolae are present in almost all cells and concentrate a wide variety of signaling molecules, receptors, transporters, and ion pumps. We have investigated the distribution of the ryanodine receptor, the Na(+)/Ca(2+) exchanger, the predominant Na(+) channel isoform rH1, and the L-type calcium channel, Ca(v)1.2, relative to the muscle-specific caveolin isoform, caveolin-3, in adult rat ventricular myocytes. Three-dimensional immunofluorescence images were deconvolved and analyzed. Caveolin-3 colocalizes with all of these molecules at the surface of the cell, but there is no significant colocalization between caveolin-3 and either the Na(+)/Ca(2+) exchanger or the Na(+) channel in the cell interior. The distribution of the surface colocalization indicates that the caveolae that colocalize with each molecule form distinct populations. This organization indicates that there are multiple populations of caveolae separable by location and occupants. In the interior of the cell, caveolin-3 shows a marked colocalization with a population of ryanodine receptors that are separate from those within the dyad. Because of their location, the signaling molecules contained within these caveolae may have preferred access to the neighboring nondyadic ryanodine receptors.
Figures




References
-
- Razani, B., S. E. Woodman, and M. P. Lisanti. 2002. Caveolae: from cell biology to animal physiology. Pharmacol. Rev. 54:431–467. - PubMed
-
- Parton, R. G. 1996. Caveolae and caveolins. Curr. Opin. Cell Biol. 8:542–548. - PubMed
-
- Song, K. S., P. E. Scherer, Z. Tang, T. Okamoto, S. Li, M. Chafel, C. Chu, S. Kohtz, and M. P. Lisanti. 1996. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. J. Biol. Chem. 271:15160–15165. - PubMed
-
- Rybin, V. O., P. W. Grabham, H. Elouardighi, and S. F. Steinberg. 2003. Caveolae-associated proteins in cardiomyocytes: caveolin-2 expression and interactions with caveolin-3. Am. J. Physiol. Heart Circ. Physiol. 285:H325–H332. - PubMed
-
- Hagiwara, Y., Y. Nishina, H. Yorifuji, and T. Kikuchi. 2002. Immunolocalization of caveolin-1 and caveolin-3 in monkey skeletal, cardiac and uterine smooth muscles. Cell Struct. Funct. 27:375–382. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

