Antiviral effect of oral administration of tenofovir disoproxil fumarate in woodchucks with chronic woodchuck hepatitis virus infection
- PMID: 15980342
- PMCID: PMC1168686
- DOI: 10.1128/AAC.49.7.2720-2728.2005
Antiviral effect of oral administration of tenofovir disoproxil fumarate in woodchucks with chronic woodchuck hepatitis virus infection
Abstract
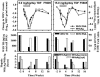
Tenofovir disoproxil fumarate (TDF) is a nucleotide analogue approved for treatment of human immunodeficiency virus (HIV) infection. TDF also has been shown in vitro to inhibit replication of wild-type hepatitis B virus (HBV) and lamivudine-resistant HBV mutants and to inhibit lamivudine-resistant HBV in patients and HBV in patients coinfected with the HIV. Data on the in vivo efficacy of TDF against wild-type virus in non-HIV-coinfected or lamivudine-naïve chronic HBV-infected patients are lacking in the published literature. The antiviral effect of oral administration of TDF against chronic woodchuck hepatitis virus (WHV) infection, an established and predictive animal model for antiviral therapy, was evaluated in a placebo-controlled, dose-ranging study (doses, 0.5 to 15.0 mg/kg of body weight/day). Four weeks of once-daily treatment with TDF doses of 0.5, 1.5, or 5.0 mg/kg/day reduced serum WHV viremia significantly (0.2 to 1.5 log reduction from pretreatment level). No effects on the levels of anti-WHV core and anti-WHV surface antibodies in serum or on the concentrations of WHV RNA or WHV antigens in the liver of treated woodchucks were observed. Individual TDF-treated woodchucks demonstrated transient declines in WHV surface antigen serum antigenemia and, characteristically, these woodchucks also had transient declines in serum WHV viremia, intrahepatic WHV replication, and hepatic expression of WHV antigens. No evidence of toxicity was observed in any of the TDF-treated woodchucks. Following drug withdrawal there was prompt recrudescence of WHV viremia to pretreatment levels. It was concluded that oral administration of TDF for 4 weeks was safe and effective in the woodchuck model of chronic HBV infection.
Figures

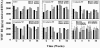
References
-
- Angus, P., R. Vaughan, S. Xiong, H. Yang, W. Delaney, C. Gibbs, C. Brosgart, D. Colledge, R. Edwards, A. Ayres, A. Bartholomeusz, and S. Locarnini. 2003. Resistance to adefovir dipivoxil therapy associated with the selection of a novel mutation in the HBV polymerase. Gastroenterology 125:292-297. - PubMed
-
- Barditch-Crovo, P., S. G. Deeks, A. Collier, S. Safrin, D. F. Coakley, M. Miller, B. P. Kearney, R. L. Coleman, P. D. Lamy, J. O. Kahn, I. McGowan, and P. S. Lietman. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 45:2733-2739. - PMC - PubMed
-
- Benhamou, Y., R. Tubiana, and V. Thibault. 2003. Tenofovir disoproxil fumarate in patients with HIV and lamivudine-resistant hepatitis B virus. N. Engl. J. Med. 348:177-178. - PubMed
-
- Bessesen, M., D. Ives, L. Condreay, S. Lawrence, and K. E. Sherman. 1999. Chronic active hepatitis B exacerbations in human immunodeficiency virus-infected patients following development of resistance to or withdrawal of lamivudine. Clin. Infect. Dis. 28:1032-1035. - PubMed
-
- Bruno, R., P. Sacchi, C. Zocchetti, V. Ciappina, M. Puoti, and G. Filice. 2003. Rapid hepatitis B virus-DNA decay in co-infected HIV-hepatitis B virus ‘e-minus’ patients with YMDD mutations after 4 weeks of tenofovir therapy. AIDS 17:783-784. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

