Constitutive soxR mutations contribute to multiple-antibiotic resistance in clinical Escherichia coli isolates
- PMID: 15980345
- PMCID: PMC1168631
- DOI: 10.1128/AAC.49.7.2746-2752.2005
Constitutive soxR mutations contribute to multiple-antibiotic resistance in clinical Escherichia coli isolates
Abstract
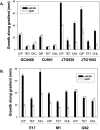
The soxRS regulon of Escherichia coli and Salmonella enterica is induced by redox-cycling compounds or nitric oxide and provides resistance to superoxide-generating agents, macrophage-generated nitric oxide, antibiotics, and organic solvents. We have previously shown that constitutive expression of soxRS can contribute to quinolone resistance in clinically relevant S. enterica. In this work, we have carried out an analysis of the mechanism of constitutive soxS expression and its role in antibiotic resistance in E. coli clinical isolates. We show that constitutive soxS expression in three out of six strains was caused by single point mutations in the soxR gene. The mutant SoxR proteins contributed to the multiple-antibiotic resistance phenotypes of the clinical strains and were sufficient to confer multiple-antibiotic resistance in a fresh genetic background. In the other three clinical isolates, we observed, for the first time, that elevated soxS expression was not due to mutations in soxR. The mechanism of such increased soxS expression remains unclear. The same E. coli clinical isolates harbored polymorphic soxR and soxS DNA sequences, also seen for the first time.
Figures


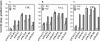
Similar articles
-
Two-stage induction of the soxRS (superoxide response) regulon of Escherichia coli.J Bacteriol. 1992 Jun;174(12):3915-20. doi: 10.1128/jb.174.12.3915-3920.1992. J Bacteriol. 1992. PMID: 1317841 Free PMC article.
-
Constitutive SoxS expression in a fluoroquinolone-resistant strain with a truncated SoxR protein and identification of a new member of the marA-soxS-rob regulon, mdtG.Antimicrob Agents Chemother. 2010 Mar;54(3):1218-25. doi: 10.1128/AAC.00944-09. Epub 2009 Dec 14. Antimicrob Agents Chemother. 2010. PMID: 20008776 Free PMC article.
-
Molecular characterization of the soxRS genes of Escherichia coli: two genes control a superoxide stress regulon.Nucleic Acids Res. 1991 Aug 25;19(16):4479-84. doi: 10.1093/nar/19.16.4479. Nucleic Acids Res. 1991. PMID: 1653416 Free PMC article.
-
Redox signaling and gene control in the Escherichia coli soxRS oxidative stress regulon--a review.Gene. 1996 Nov 7;179(1):53-7. doi: 10.1016/s0378-1119(96)00329-0. Gene. 1996. PMID: 8955629 Review.
-
Sensing and protecting against superoxide stress in Escherichia coli--how many ways are there to trigger soxRS response?Redox Rep. 2000;5(5):287-93. doi: 10.1179/135100000101535825. Redox Rep. 2000. PMID: 11145103 Review.
Cited by
-
Functional analysis of bacterial genes accidentally packaged in rhizospheric phageome of the wild plant species Abutilon fruticosum.Saudi J Biol Sci. 2023 Oct;30(10):103789. doi: 10.1016/j.sjbs.2023.103789. Epub 2023 Aug 26. Saudi J Biol Sci. 2023. PMID: 37680975 Free PMC article.
-
Antibiotic tolerance is associated with a broad and complex transcriptional response in E. coli.Sci Rep. 2021 Mar 17;11(1):6112. doi: 10.1038/s41598-021-85509-7. Sci Rep. 2021. PMID: 33731833 Free PMC article.
-
Clinical and molecular characteristics of carbapenem non-susceptible Escherichia coli: A nationwide survey from Oman.PLoS One. 2020 Oct 9;15(10):e0239924. doi: 10.1371/journal.pone.0239924. eCollection 2020. PLoS One. 2020. PMID: 33036018 Free PMC article.
-
Role of efflux pumps, their inhibitors, and regulators in colistin resistance.Front Microbiol. 2023 Aug 4;14:1207441. doi: 10.3389/fmicb.2023.1207441. eCollection 2023. Front Microbiol. 2023. PMID: 37601369 Free PMC article. Review.
-
Multiple regulatory pathways associated with high-level ciprofloxacin and multidrug resistance in Salmonella enterica serovar enteritidis: involvement of RamA and other global regulators.Antimicrob Agents Chemother. 2009 Mar;53(3):1080-7. doi: 10.1128/AAC.01005-08. Epub 2008 Dec 22. Antimicrob Agents Chemother. 2009. PMID: 19104017 Free PMC article.
References
-
- Chander, M., and B. Demple. 2004. Functional analysis of SoxR residues affecting transduction of oxidative stress signals into gene expression. J. Biol. Chem. 279:41603-41610. - PubMed
-
- Changela, A., K. Chen, Y. Xue, J. Holschen, C. E. Outten, T. V. O'Halloran, and A. Mondragon. 2003. Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science 301:1383-1387. - PubMed
-
- Dennesen, P. J., M. J. Bonten, and R. A. Weinstein. 1998. Multiresistant bacteria as a hospital epidemic problem. Ann. Med. 30:176-185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

