Imipenem for treatment of tuberculosis in mice and humans
- PMID: 15980354
- PMCID: PMC1168716
- DOI: 10.1128/AAC.49.7.2816-2821.2005
Imipenem for treatment of tuberculosis in mice and humans
Abstract
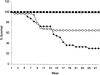
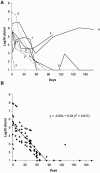
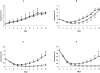
Chemotherapy of tuberculosis caused by multiple-drug-resistant (MDR) strains is problematic because of choices limited to relatively inefficacious and toxic drugs. Some beta-lactam antibiotics are active against Mycobacterium tuberculosis in vitro. We investigated the efficacy of imipenem in a mouse model of tuberculosis and in humans with MDR tuberculosis. Mice infected with M. tuberculosis strain H37Rv were treated with isoniazid or imipenem. Residual organisms in lung and spleen and survival of imipenem-treated mice were compared to those of untreated or isoniazid-treated mice. Ten patients with MDR tuberculosis also were treated with imipenem in combination with other first- or second-line agents; elimination of M. tuberculosis from sputum samples was measured by quantitative culture. Although it was less effective than isoniazid, imipenem significantly reduced the numbers of M. tuberculosis organisms in lungs and spleens and improved survival of mice. Eight of 10 patients with numerous risk factors for poor outcomes responded to imipenem combination therapy with conversion of cultures to negative. Seven remained culture-negative off of therapy. There were two deaths, one of which was due to active tuberculosis. Organisms were eliminated from the sputa of responders at a rate of 0.35 log10 CFU/ml/week. Relapse upon withdrawal of imipenem and development of resistance to imipenem in a nonresponder suggest that imipenem exerts antimycobacterial activity in humans infected with M. tuberculosis. Imipenem had antimycobacterial activity both in a mouse model and in humans at high risk for failure of treatment for MDR tuberculosis.
Figures
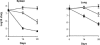
References
-
- Chambers, H. F., T. Kocagoz, T. Sipit, J. Turner, and P. C. Hopewell. 1998. Activity of amoxicillin/clavulanate in patients with tuberculosis. Clin. Infect. Dis. 26:874-877. - PubMed
-
- Finch, R. 1986. Beta-lactam antibiotics and mycobacteria. J. Antimicrob. Chemother. 18:6-8. - PubMed
-
- Goble, M., M. D. Iseman, L. A. Madsen, D. Waite, L. Ackerson, and C. R. Horsburgh, Jr. 1993. Treatment of 171 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. N. Engl. J. Med. 328:527-532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

