Antimicrobial and chemoattractant activity, lipopolysaccharide neutralization, cytotoxicity, and inhibition by serum of analogs of human cathelicidin LL-37
- PMID: 15980359
- PMCID: PMC1168709
- DOI: 10.1128/AAC.49.7.2845-2850.2005
Antimicrobial and chemoattractant activity, lipopolysaccharide neutralization, cytotoxicity, and inhibition by serum of analogs of human cathelicidin LL-37
Abstract
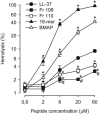
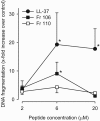
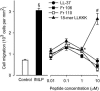
Antimicrobial peptides have been evaluated in vitro and in vivo as alternatives to conventional antibiotics. Apart from being antimicrobial, the native human cathelicidin-derived peptide LL-37 (amino acids [aa] 104 to 140 of the human cathelicidin antimicrobial peptide) also binds and neutralizes bacterial lipopolysaccharide (LPS) and might therefore have beneficial effects in the treatment of septic shock. However, clinical trials have been hampered by indications of toxic effects of LL-37 on mammalian cells and evidence that its antimicrobial effects are inhibited by serum. For the present study, LL-37 was compared to two less hydrophobic fragments obtained by N-terminal truncation, named 106 (aa 106 to 140) and 110 (aa 110 to 140), and to a previously described more hydrophobic variant, the 18-mer LLKKK, concerning antimicrobial properties, lipopolysaccharide neutralization, toxicity against human erythrocytes and cultured vascular smooth muscle cells, chemotactic activity, and inhibition by serum. LL-37, fragments 106 and 110, and the 18-mer LLKKK inhibited the growth of Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Candida albicans in a radial diffusion assay, inhibited lipopolysaccharide-induced vascular nitric oxide production, and attracted neutrophil granulocytes similarly. While fragments 106 and 110 caused less hemolysis and DNA fragmentation in cultured cells than did LL-37, the 18-mer LLKKK induced severe hemolysis. The antibacterial effect of fragments 106 and 110 was not affected by serum, while the effect of LL-37 was reduced. We concluded that the removal of N-terminal hydrophobic amino acids from LL-37 decreases its cytotoxicity as well as its inhibition by serum without negatively affecting its antimicrobial or LPS-neutralizing action. Such LL-37-derived peptides may thus be beneficial for the treatment of patients with sepsis.
Figures
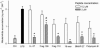
Similar articles
-
Augmentation of the lipopolysaccharide-neutralizing activities of human cathelicidin CAP18/LL-37-derived antimicrobial peptides by replacement with hydrophobic and cationic amino acid residues.Clin Diagn Lab Immunol. 2002 Sep;9(5):972-82. doi: 10.1128/cdli.9.5.972-982.2002. Clin Diagn Lab Immunol. 2002. PMID: 12204946 Free PMC article.
-
In silico identification and biological evaluation of antimicrobial peptides based on human cathelicidin LL-37.Antimicrob Agents Chemother. 2006 Sep;50(9):2983-9. doi: 10.1128/AAC.01583-05. Antimicrob Agents Chemother. 2006. PMID: 16940092 Free PMC article.
-
Short KR-12 analogs designed from human cathelicidin LL-37 possessing both antimicrobial and antiendotoxic activities without mammalian cell toxicity.J Pept Sci. 2013 Nov;19(11):700-7. doi: 10.1002/psc.2552. Epub 2013 Sep 17. J Pept Sci. 2013. PMID: 24105706
-
High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments.Biochim Biophys Acta. 2014 Sep;1838(9):2160-72. doi: 10.1016/j.bbamem.2014.01.016. Epub 2014 Jan 23. Biochim Biophys Acta. 2014. PMID: 24463069 Free PMC article. Review.
-
Antibacterial function of the human cathelicidin-18 peptide (LL-37) between theory and practice.Protein Pept Lett. 2014;21(12):1247-56. Protein Pept Lett. 2014. PMID: 25101632 Review.
Cited by
-
Beneficial impact of cathelicidin on hypersensitivity pneumonitis treatment-In vivo studies.PLoS One. 2021 May 17;16(5):e0251237. doi: 10.1371/journal.pone.0251237. eCollection 2021. PLoS One. 2021. PMID: 33999928 Free PMC article.
-
Human antimicrobial peptide LL-37 is present in atherosclerotic plaques and induces death of vascular smooth muscle cells: a laboratory study.BMC Cardiovasc Disord. 2006 Dec 20;6:49. doi: 10.1186/1471-2261-6-49. BMC Cardiovasc Disord. 2006. PMID: 17181861 Free PMC article.
-
Antimicrobial Peptides: From Design to Clinical Application.Antibiotics (Basel). 2022 Mar 6;11(3):349. doi: 10.3390/antibiotics11030349. Antibiotics (Basel). 2022. PMID: 35326812 Free PMC article. Review.
-
Cathelicidin-deficient mice exhibit increased survival and upregulation of key inflammatory response genes following cecal ligation and puncture.J Mol Med (Berl). 2017 Sep;95(9):995-1003. doi: 10.1007/s00109-017-1555-z. Epub 2017 Jun 16. J Mol Med (Berl). 2017. PMID: 28623379
-
The roles of vitamin D and cathelicidin in type 1 diabetes susceptibility.Endocr Connect. 2021 Jan;10(1):R1-R12. doi: 10.1530/EC-20-0484. Endocr Connect. 2021. PMID: 33263562 Free PMC article. Review.
References
-
- Agerberth, B., J. Charo, J. Werr, B. Olsson, F. Idali, L. Lindbom, R. Kiessling, H. Jörnvall, H. Wigzell, and G. H. Gudmundsson. 2000. The human antimicrobial and chemotactic peptides LL-37 and α-defensins are expressed by specific lymphocyte and monocyte populations. Blood 96:3086-3093. - PubMed
-
- Ciornei, C. D., A. Egesten, and M. Bodelsson. 2003. Effects of human cathelicidin antimicrobial peptide LL-37 on lipopolysaccharide-induced nitric oxide release from rat aorta in vitro. Acta Anaesthesiol. Scand. 47:213-220. - PubMed
-
- Cohen, J. 2002. The immunopathogenesis of sepsis. Nature 420:885-891. - PubMed
-
- Fleming, I., G. A. Gray, G. Julou-Schaeffer, J. R. Parratt, and J. C. Stoclet. 1990. Incubation with endotoxin activates the l-arginine pathway in vascular tissue. Biochem. Biophys. Res. Commun. 171:562-568. - PubMed
-
- Fukomoto, K., I. Nagaoka, A. Yamataka, H. Kobayashi, T. Yanai, Y. Kato, and T. Miyano. 2005. Effect of antibacterial cathelicidin peptide CAP18/LL-37 on sepsis in neonatal rats. Pediatr. Surg. Int. 121:20-24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

