I-Mutant2.0: predicting stability changes upon mutation from the protein sequence or structure
- PMID: 15980478
- PMCID: PMC1160136
- DOI: 10.1093/nar/gki375
I-Mutant2.0: predicting stability changes upon mutation from the protein sequence or structure
Abstract
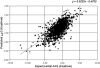
I-Mutant2.0 is a support vector machine (SVM)-based tool for the automatic prediction of protein stability changes upon single point mutations. I-Mutant2.0 predictions are performed starting either from the protein structure or, more importantly, from the protein sequence. This latter task, to the best of our knowledge, is exploited for the first time. The method was trained and tested on a data set derived from ProTherm, which is presently the most comprehensive available database of thermodynamic experimental data of free energy changes of protein stability upon mutation under different conditions. I-Mutant2.0 can be used both as a classifier for predicting the sign of the protein stability change upon mutation and as a regression estimator for predicting the related DeltaDeltaG values. Acting as a classifier, I-Mutant2.0 correctly predicts (with a cross-validation procedure) 80% or 77% of the data set, depending on the usage of structural or sequence information, respectively. When predicting DeltaDeltaG values associated with mutations, the correlation of predicted with expected/experimental values is 0.71 (with a standard error of 1.30 kcal/mol) and 0.62 (with a standard error of 1.45 kcal/mol) when structural or sequence information are respectively adopted. Our web interface allows the selection of a predictive mode that depends on the availability of the protein structure and/or sequence. In this latter case, the web server requires only pasting of a protein sequence in a raw format. We therefore introduce I-Mutant2.0 as a unique and valuable helper for protein design, even when the protein structure is not yet known with atomic resolution.
Availability: http://gpcr.biocomp.unibo.it/cgi/predictors/I-Mutant2.0/I-Mutant2.0.cgi.
Figures

References
-
- Casadio R., Compiani M., Fariselli P., Vivarelli F. Predicting free energy contributions to the conformational stability of folded proteins from the residue sequence with radial basis function networks. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1995;3:81–88. - PubMed
-
- Gilis D., Rooman M. Predicting protein stability changes upon mutation using database-derived potentials: solvent accessibility determines the importance of local versus non-local interactions along the sequence. J. Mol. Biol. 1997;272:276–290. - PubMed
-
- Guerois R., Nielsen J.E., Serrano L. Predicting changes in the stability of proteins and protein complexes: a study of more than 1000 mutations. J. Mol. Biol. 2002;320:369–387. - PubMed
-
- Khatun J., Khare S.D., Dokholyan N.V. Can contact potentials reliably predict stability of proteins? J. Mol. Biol. 2004;336:1223–1238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

