Nonstimulatory peptides contribute to antigen-induced CD8-T cell receptor interaction at the immunological synapse
- PMID: 15980863
- PMCID: PMC1352171
- DOI: 10.1038/ni1220
Nonstimulatory peptides contribute to antigen-induced CD8-T cell receptor interaction at the immunological synapse
Abstract
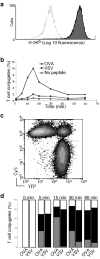
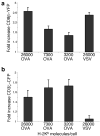
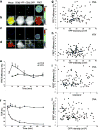
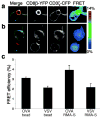
It is unclear if the interaction between CD8 and the T cell receptor (TCR)-CD3 complex is constitutive or antigen induced. Here, fluorescence resonance energy transfer microscopy between fluorescent chimeras of CD3zeta and CD8beta showed that this interaction was induced by antigen recognition in the immunological synapse. Nonstimulatory endogenous or exogenous peptides presented simultaneously with antigenic peptides increased the CD8-TCR interaction. This finding indicates that the interaction between the intracellular regions of a TCR-CD3 complex recognizing its cognate peptide-major histocompatibility complex (MHC) antigen, and CD8 (plus the kinase Lck), is enhanced by a noncognate CD8-MHC interaction. Thus, the interaction of CD8 with a nonstimulatory peptide-MHC complex helps mediate T cell recognition of antigen, improving the coreceptor function of CD8.
Conflict of interest statement
Competing interests statement
The authors declare that they have no competing financial interests.
Figures

References
-
- Davis MM, et al. Ligand recognition by αβ T cell receptors. Annu Rev Immunol. 1998;16:523–534. - PubMed
-
- Gascoigne NRJ, Zal T, Alam SM. T-cell receptor binding kinetics in T-cell development and activation. Exp Rev Mol Med. 2001 Feb 12;2001:1–17. ( http://www.expertreviews.org/01002502h.htm) - PubMed
-
- Zamoyska R. CD4 and CD8: Modulators of T-cell receptor recognition of antigen and of immune responses? Curr Opin Immunol. 1998;10:82–87. - PubMed
-
- Potter TA, Rajan TV, Dick RF, 2nd, Bluestone JA. Substitution at residue 227 of H-2 class I molecules abrogates recognition by CD8-dependent, but not CD8-independent, cytotoxic T lymphocytes. Nature. 1989;337:73–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
