Evaluation of benzyltetrahydroisoquinolines as ligands for neuronal nicotinic acetylcholine receptors
- PMID: 15980871
- PMCID: PMC1576253
- DOI: 10.1038/sj.bjp.0706307
Evaluation of benzyltetrahydroisoquinolines as ligands for neuronal nicotinic acetylcholine receptors
Abstract
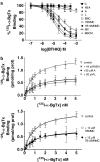
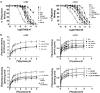
Effects of derivatives of coclaurine (C), which mimic the 'eastern' or the nonquaternary halves of the alkaloids tetrandrine or d-tubocurarine, respectively, both of which are inhibitors of nicotinic acetylcholine receptors (nACh), were examined on recombinant, human alpha7, alpha4beta2 and alpha4beta4 nACh receptors expressed in Xenopus oocytes and clonal cell lines using two-electrode voltage clamping and radioligand binding techniques. In this limited series, Cs have higher affinity and are most potent at alpha4 subunit-containing-nACh receptors and least potent at homomeric alpha7 receptors, and this trend is very marked for the N-unsubstituted C and its O,O'-bisbenzyl derivative. 7-O-Benzyl-N-methylcoclaurine (BBCM) and its 12-O-methyl derivative showed the highest affinities and potencies at all three receptor subtypes, and this suggests that lipophilicity at C7 and/or C12 increases potency. Laudanosine and armepavine (A) were noncompetitive and voltage-dependent inhibitors of alpha7, alpha4beta2 or alpha4beta4 receptors, but the bulkier C7-benzylated 7BNMC (7-O-benzyl-N-methylcoclaurine) and 7B12MNMC (7-O-benzyl-N,12-O-dimethyl coclaurine) were voltage-independent, noncompetitive inhibitors of nACh receptors. Voltage-dependence was also lost on going from A to its N-ethyl analogue. These studies suggest that C derivatives may be useful tools for studies characterising the antagonist and ion channel sites on human alpha7, alpha4beta2 or alpha4beta4 nACh receptors and for revealing structure-function relationships for nACh receptor antagonists.
Figures




References
-
- ASTLES P.C., BAKER R.S., BOOT J.R., BROAD L.M., DELL C.P., KEENAN M. Recent progress in the development of subtype selective nicotinic acetylcholine receptor ligands. Curr. Drug Targets – CNS Neurol. Disord. 2002;1:337–348. - PubMed
-
- BUCK K.T.Bisbenzylisoquinolines The Alkaloids 1987New York: Academic Press; 1–222.ed. Brossi, A. pp
-
- CACHELIN A.B., RUST G. Unusual pharmacology of (+)-tubocurarine with rat neuronal nicotinic acetylcholine receptors containing β4 subunits. Mol. Pharmacol. 1994;46:1168–1174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

