Novel properties of a mouse gamma-aminobutyric acid transporter (GAT4)
- PMID: 15981712
- PMCID: PMC3009668
- DOI: 10.1007/s00232-004-0732-5
Novel properties of a mouse gamma-aminobutyric acid transporter (GAT4)
Abstract
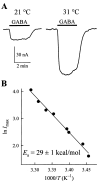
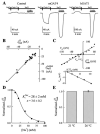
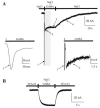
We expressed the mouse gamma-aminobutyric acid (GABA) transporter GAT4 (homologous to rat/ human GAT-3) in Xenopus laevis oocytes and examined its functional and pharmacological properties by using electrophysiological and tracer uptake methods. In the coupled mode of transport (Na+/ Cl-/GABA cotransport), there was tight coupling between charge flux and GABA flux across the plasma membrane (2 charges/GABA). Transport was highly temperature-dependent with a temperature coefficient (Q10) of 4.3. The GAT4 turnover rate (1.5 s(-l); -50 mV, 21 degrees C) and temperature dependence suggest physiological turnover rates of 15-20 s(-1). No uncoupled current was observed in the presence of Na+. In the absence of external Na+, GAT4 exhibited two distinct uncoupled currents. (i) A Cl- leak current (ICl(leak)) was observed when Na+ was replaced with choline or tetraethylammonium. The reversal potential of (ICl(leak)) followed the Cl- Nernst potential. (ii) A Li+ leak current (ILi(leak)) was observed when Na+ was replaced with Li+. Both leak currents were inhibited by Na+, and both were temperature-independent (Q10 approximately 1). The two leak modes appeared not to coexist, as Li+ inhibited (ICl(leak)). The results suggest the existence of cation- and anion-selective channel-like pathways in GAT4. Flufenamic acid inhibited GAT4 Na+/Cl-/GABA cotransport, ILi(leak), and ICl(leak), (Ki approximately 30 microM), and the voltage-induced presteady-state charge movements (Ki approximately 440 microM). Flufenamic acid exhibited little or no selectivity for GAT1, GAT2, or GAT3. Sodium and GABA concentration jicroumps revealed that slow Na+ binding to the transporter is followed by rapid GABA-induced translocation of the ligands across the plasma membrane. Thus, Na+ binding and associated conformational changes constitute the rate-limiting steps in the transport cycle.
Figures

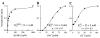







Similar articles
-
Effect of sodium lithium and proton concentrations on the electrophysiological properties of the four mouse GABA transporters expressed in Xenopus oocytes.Neurochem Int. 2003 Sep-Oct;43(4-5):431-43. doi: 10.1016/s0197-0186(03)00032-9. Neurochem Int. 2003. PMID: 12742089
-
GAT1 (GABA:Na+:Cl-) cotransport function. Steady state studies in giant Xenopus oocyte membrane patches.J Gen Physiol. 1999 Sep;114(3):429-44. doi: 10.1085/jgp.114.3.429. J Gen Physiol. 1999. PMID: 10469733 Free PMC article.
-
Presteady-state and steady-state kinetics and turnover rate of the mouse gamma-aminobutyric acid transporter (mGAT3).J Membr Biol. 2002 Nov 1;190(1):57-73. doi: 10.1007/s00232-002-1024-6. J Membr Biol. 2002. PMID: 12422272
-
Molecular characterization of four pharmacologically distinct gamma-aminobutyric acid transporters in mouse brain [corrected].J Biol Chem. 1993 Jan 25;268(3):2106-12. J Biol Chem. 1993. PMID: 8420981
-
Passive water and ion transport by cotransporters.J Physiol. 1999 Jul 1;518(Pt 1):195-202. doi: 10.1111/j.1469-7793.1999.0195r.x. J Physiol. 1999. PMID: 10373701 Free PMC article.
Cited by
-
The solute carrier 6 family of transporters.Br J Pharmacol. 2012 Sep;167(2):256-78. doi: 10.1111/j.1476-5381.2012.01975.x. Br J Pharmacol. 2012. PMID: 22519513 Free PMC article. Review.
-
Temperature dependence of steady-state and presteady-state kinetics of a type IIb Na+/P i cotransporter.J Membr Biol. 2007 Feb;215(2-3):81-92. doi: 10.1007/s00232-007-9008-1. Epub 2007 Apr 19. J Membr Biol. 2007. PMID: 17443384
-
Amino Acid Transporter SLC6A14 (ATB0,+) - A Target in Combined Anti-cancer Therapy.Front Cell Dev Biol. 2020 Oct 21;8:594464. doi: 10.3389/fcell.2020.594464. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33195271 Free PMC article. Review.
-
Functional expression of γ-amino butyric acid transporter 2 in human and guinea pig airway epithelium and smooth muscle.Am J Respir Cell Mol Biol. 2011 Aug;45(2):332-9. doi: 10.1165/rcmb.2010-0177OC. Epub 2010 Nov 5. Am J Respir Cell Mol Biol. 2011. PMID: 21057105 Free PMC article.
-
Cl⁻ homeodynamics in gap junction-coupled astrocytic networks on activation of GABAergic synapses.J Physiol. 2013 Aug 15;591(16):3901-17. doi: 10.1113/jphysiol.2013.257162. Epub 2013 Jun 3. J Physiol. 2013. PMID: 23732644 Free PMC article.
References
-
- Binda F, Bossi E, Giovannardi S, Forlani G, Peres A. Temperature effects on the presteady-state and transport-associated currents of GABA cotransporter rGAT1. FEBS Lett. 2002;512:303–307. - PubMed
-
- Bismuth Y, Kavanaugh MP, Kanner BI. Tyrosine 140 of the γ-aminobutyric acid transporter GAT–1 plays a critical role in neurotransmitter recognition. J. Biol. Chem. 1997;272:16096–16102. - PubMed
-
- Borden LA. GABA transporter heterogeneity: Pharmacology and cellular localization. Neurochem. Int. 1996;29:335–356. - PubMed
-
- Borden LA, Dhar TG, Smith K, Branchek TA, Gluchowshi C, Weinshank R. Cloning of the human homologue of the GABA transporter GAT-3 and identification of a novel inhibitor with selectivity for this site. Receptors Channels. 1994;2:207–312. - PubMed
-
- Cammack JN, Rakhilin SV, Schwartz EA. A GABA transporter operates asymmetrically and with variable stoichiometry. Neuron. 1994;13:949–960. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

