Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2)
- PMID: 15983030
- PMCID: PMC8062222
- DOI: 10.1074/jbc.M505111200
Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2)
Abstract
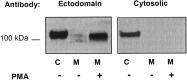
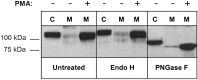
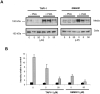
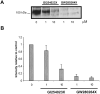
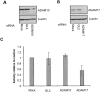
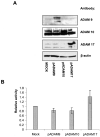
Angiotensin-converting enzyme-2 (ACE2) is a critical regulator of heart function and a cellular receptor for the causative agent of severe-acute respiratory syndrome (SARS), SARS-CoV (coronavirus). ACE2 is a type I transmembrane protein, with an extracellular N-terminal domain containing the active site and a short intracellular C-terminal tail. A soluble form of ACE2, lacking its cytosolic and transmembrane domains, has been shown to block binding of the SARS-CoV spike protein to its receptor. In this study, we examined the ability of ACE2 to undergo proteolytic shedding and investigated the mechanisms responsible for this shedding event. We demonstrated that ACE2, heterologously expressed in HEK293 cells and endogenously expressed in Huh7 cells, undergoes metalloproteinase-mediated, phorbol ester-inducible ectodomain shedding. By using inhibitors with differing potency toward different members of the ADAM (a disintegrin and metalloproteinase) family of proteases, we identified ADAM17 as a candidate mediator of stimulated ACE2 shedding. Furthermore, ablation of ADAM17 expression using specific small interfering RNA duplexes reduced regulated ACE2 shedding, whereas overexpression of ADAM17 significantly increased shedding. Taken together, these data provided direct evidence for the involvement of ADAM17 in the regulated ectodomain shedding of ACE2. The identification of ADAM17 as the protease responsible for ACE2 shedding may provide new insight into the physiological roles of ACE2.
Figures

References
-
- Erdos E.G. Circ. Res. 1975;36:247–255. - PubMed
-
- Ehlers M.R.W., Riordan J.F. Biochemistry. 1989;28:5311–5318. - PubMed
-
- Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. J. Biol. Chem. 2000;275:33238–33243. - PubMed
-
- Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R.E., Acton S. Circ. Res. 2000;87:e1–e9. - PubMed
-
- Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E., Oliveira-dos-Santos A.J., da Costa J., Zhang L., Pei Y., Scholey J., Ferrario C.M., Manoukian A.S., Chappell M.C., Backx P.H., Yagil Y., Penninger J.M. Nature. 2002;417:822–828. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

