TGF-{beta} maintains dormancy of prostatic stem cells in the proximal region of ducts
- PMID: 15983059
- PMCID: PMC2171389
- DOI: 10.1083/jcb.200412015
TGF-{beta} maintains dormancy of prostatic stem cells in the proximal region of ducts
Abstract
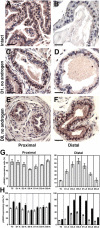
We have previously shown that prostatic stem cells are located in the proximal region of mouse prostatic ducts. Here, we show that this region responds differently to transforming growth factor (TGF)-beta than the distal ductal region and that under physiological conditions androgens and TGF-beta are crucial overall regulators of prostatic tissue homeostasis. This conclusion is supported by the observations showing that high levels of TGF-beta signaling are present in the quiescent proximal region of ducts in an androgen-replete animal and that cells in this region overexpress Bcl-2, which protects them from apoptosis. Moreover, androgen ablation reverses the proximal-distal TGF-beta signaling gradient, leading to an increase in TGF-beta signaling in the unprotected distal region (low Bcl-2 expression). This reversal of TGF-beta-mediated signaling accompanies apoptosis of cells in the distal region and gland involution after androgen withdrawal. A physiological TGF-beta signaling gradient (high proximally and low distally) and its functional correlates are restored after androgen replenishment. In addition to highlighting the regulatory role of androgens and TGF-beta, these findings may have important implications for the deregulation of the stem cell compartment in the etiology of proliferative prostatic diseases.
Figures







References
-
- Abe, M., J.G. Harpel, C.N. Metz, I. Nunes, D.J. Loskutoff, and D.B. Rifkin. 1994. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal. Biochem. 216:276–284. - PubMed
-
- Adams, J.M., and S. Cory. 1998. The Bcl-2 protein family: arbiters of cell survival. Science. 281:1322–1326. - PubMed
-
- Beachy, P.A., S.S. Karhadkar, and D.M. Berman. 2004. Tissue repair and stem cell renewal in carcinogenesis. Nature. 432:324–331. - PubMed
-
- Bruckheimer, E.M., and N. Kyprianou. 2002. Bcl-2 antagonizes the combined apoptotic effect of transforming growth factor-beta and dihydrotestosterone in prostate cancer cells. Prostate. 53:133–142. - PubMed
-
- Cashman, J.D., A.C. Eaves, and C.J. Eaves. 1992. Granulocyte-macrophage colony-stimulating factor modulation of the inhibitory effect of transforming growth factor-beta on normal and leukemic human hematopoietic progenitor cells. Leukemia. 6:886–892. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

