Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration
- PMID: 15983386
- PMCID: PMC1172259
- DOI: 10.1073/pnas.0503437102
Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration
Abstract
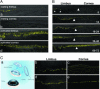
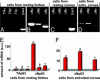
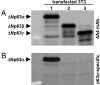
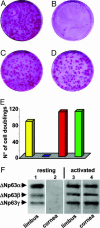
The p63 gene generates transactivating and N-terminally truncated transcripts (DeltaNp63) initiated by different promoters. Alternative splicing gives rise to three different C termini, designated alpha, beta, and gamma. In the ocular epithelium, the corneal stem cells, which are segregated in the basal layer of the limbus, contain the alpha isoform but not beta or gamma. Holoclones derived from the limbus are rich in alpha, meroclones contain little, and paraclones contain none. In normal resting corneal epithelium, p63 of all isoforms is absent. Upon corneal wounding, cells originating from the limbus and containing alpha migrate progressively through the epithelium of the peripheral and central cornea. In the absence of an attached limbus, no alpha isoform appears in the corneal epithelium. When migrating cells containing the alpha isoform appear in the wounded corneal epithelium, they are confined to the basal layer, but the suprabasal cells, not only of the cornea but of the limbus as well, contain mRNA encoding beta and gamma. These data support the concept that the alpha isoform of p63 is necessary for the maintenance of the proliferative potential of limbal stem cells and their ability to migrate over the cornea. The beta and gamma isoforms, being suprabasal and virtually absent from the resting limbus, are not stem cell markers but are likely to play a role in epithelial differentiation specifically during the process of corneal regeneration.
Figures



Similar articles
-
Acute wound healing in the human central corneal epithelium appears to be independent of limbal stem cell influence.Invest Ophthalmol Vis Sci. 2008 Dec;49(12):5279-86. doi: 10.1167/iovs.07-1260. Epub 2008 May 30. Invest Ophthalmol Vis Sci. 2008. PMID: 18515566
-
Regulation of limbal keratinocyte proliferation and differentiation by TAp63 and DeltaNp63 transcription factors.Invest Ophthalmol Vis Sci. 2005 Sep;46(9):3102-8. doi: 10.1167/iovs.05-0051. Invest Ophthalmol Vis Sci. 2005. PMID: 16123408
-
Epithelial regeneration after limbus-to-limbus debridement. Expression of alpha-enolase in stem and transient amplifying cells.Invest Ophthalmol Vis Sci. 1995 Jun;36(7):1336-43. Invest Ophthalmol Vis Sci. 1995. PMID: 7775111
-
Corneal stem cells in review.Wound Repair Regen. 2001 Nov-Dec;9(6):483-94. doi: 10.1046/j.1524-475x.2001.00483.x. Wound Repair Regen. 2001. PMID: 11896990 Review.
-
Identification and characterization of limbal stem cells.Exp Eye Res. 2005 Sep;81(3):247-64. doi: 10.1016/j.exer.2005.02.016. Exp Eye Res. 2005. PMID: 16051216 Review.
Cited by
-
Bio-printing method as a novel approach to obtain a fibrin scaffold settled by limbal epithelial cells for corneal regeneration.Sci Rep. 2024 Oct 7;14(1):23352. doi: 10.1038/s41598-024-73383-y. Sci Rep. 2024. PMID: 39375390 Free PMC article.
-
From discovery to approval of an advanced therapy medicinal product-containing stem cells, in the EU.Regen Med. 2016 Jun;11(4):407-20. doi: 10.2217/rme-2015-0051. Epub 2016 Apr 19. Regen Med. 2016. PMID: 27091398 Free PMC article. Review.
-
Single-keratinocyte transcriptomic analyses identify different clonal types and proliferative potential mediated by FOXM1 in human epidermal stem cells.Nat Commun. 2021 May 4;12(1):2505. doi: 10.1038/s41467-021-22779-9. Nat Commun. 2021. PMID: 33947848 Free PMC article.
-
Synthetic vs natural scaffolds for human limbal stem cells.Croat Med J. 2015 Jun;56(3):246-56. doi: 10.3325/cmj.2015.56.246. Croat Med J. 2015. PMID: 26088849 Free PMC article.
-
Bevacizumab Induces Upregulation of Keratin 3 and VEGFA in Human Limbal Epithelial Cells in Vitro.J Clin Med. 2019 Nov 9;8(11):1925. doi: 10.3390/jcm8111925. J Clin Med. 2019. PMID: 31717500 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

