Estrogen regulation of cell proliferation and distribution of estrogen receptor-alpha in the brains of adult female prairie and meadow voles
- PMID: 15984004
- PMCID: PMC3962047
- DOI: 10.1002/cne.20638
Estrogen regulation of cell proliferation and distribution of estrogen receptor-alpha in the brains of adult female prairie and meadow voles
Abstract
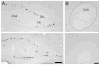
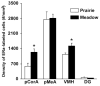
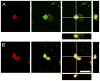
Adult female prairie (Microtus ochrogaster) and meadow (M. pennsylvanicus) voles were compared to examine neural cell proliferation and the effects of estrogen manipulation on cell proliferation in the amygdala, ventromedial hypothalamus (VMH), and dentate gyrus of the hippocampus (DG). Unlike prior studies, our study focused on the amygdala and VMH, because they are involved in social behaviors and may underlie behavioral differences between the species. Meadow voles had a higher density of cells labeled with the cell proliferation marker 5-bromo-2'-deoxyuridine (BrdU) in the amygdala and DG than did prairie voles. Treatment with estradiol benzoate (EB) for 3 days increased the density of BrdU-labeled cells in the amygdala, particularly in the posterior cortical (pCorA) and medial (pMeA) nuclei, in meadow, but not prairie, voles. Furthermore, the majority of the BrdU-labeled cells in the pCorA and pMeA displayed either a neuronal or a glial progenitor phenotype, but no species or treatment differences were found in the percentage of neuronal or glial progenitor cells. To understand better estrogen's effects on adult neurogenesis, we also examined estrogen receptor-alpha (ERalpha) distribution. Meadow voles had more ERalpha-labeled cells in the pCorA and VMH, but not in the pMeA or DG, than did prairie voles. In addition, more than one-half of the BrdU-labeled cells in the amygdala of both species coexpressed ERalpha labeling. Together, these data indicate that estrogen alters cell proliferation in a species- and region-specific manner, and some of these effects may lie in the specific localization of estrogen receptors in the adult vole brain.
(c) 2005 Wiley-Liss, Inc.
Figures


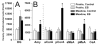


Similar articles
-
Species differences in behavior and cell proliferation/survival in the adult brains of female meadow and prairie voles.Neuroscience. 2016 Feb 19;315:259-70. doi: 10.1016/j.neuroscience.2015.12.026. Epub 2015 Dec 19. Neuroscience. 2016. PMID: 26708743 Free PMC article.
-
Newly proliferated cells in the adult male amygdala are affected by gonadal steroid hormones.J Neurobiol. 2003 Dec;57(3):257-69. doi: 10.1002/neu.10273. J Neurobiol. 2003. PMID: 14608662
-
Social environment alters central distribution of estrogen receptor alpha in juvenile prairie voles.Physiol Behav. 2009 Sep 7;98(3):296-301. doi: 10.1016/j.physbeh.2009.06.005. Epub 2009 Jun 17. Physiol Behav. 2009. PMID: 19539635 Free PMC article.
-
Frank Beach award winner: Neuroendocrinology of group living.Horm Behav. 2019 Jan;107:67-75. doi: 10.1016/j.yhbeh.2018.11.002. Epub 2018 Dec 17. Horm Behav. 2019. PMID: 30439353 Free PMC article. Review.
-
Sexually dimorphic spatial learning in meadow voles Microtus pennsylvanicus and deer mice Peromyscus maniculatus.J Exp Biol. 1996 Jan;199(Pt 1):195-200. doi: 10.1242/jeb.199.1.195. J Exp Biol. 1996. PMID: 8576690 Review.
Cited by
-
The Extract of Fructus Psoraleae Promotes Viability and Cartilaginous Formation of Rat Chondrocytes In Vitro.Evid Based Complement Alternat Med. 2016;2016:2057631. doi: 10.1155/2016/2057631. Epub 2016 Nov 23. Evid Based Complement Alternat Med. 2016. PMID: 27994628 Free PMC article.
-
Androgenic and oestrogenic influences on tyrosine hydroxylase-immunoreactive cells of the prairie vole medial amygdala and bed nucleus of the stria terminalis.J Neuroendocrinol. 2010 Apr;22(4):217-25. doi: 10.1111/j.1365-2826.2010.01958.x. Epub 2010 Jan 27. J Neuroendocrinol. 2010. PMID: 20136687 Free PMC article.
-
Socially modulated cell proliferation is independent of gonadal steroid hormones in the brain of the adult green treefrog (Hyla cinerea).Brain Behav Evol. 2012;79(3):170-80. doi: 10.1159/000335037. Epub 2012 Jan 20. Brain Behav Evol. 2012. PMID: 22269468 Free PMC article.
-
μ-Opioid receptors within subregions of the striatum mediate pair bond formation through parallel yet distinct reward mechanisms.J Neurosci. 2013 May 22;33(21):9140-9. doi: 10.1523/JNEUROSCI.4123-12.2013. J Neurosci. 2013. PMID: 23699524 Free PMC article.
-
Sex-specific and shared expression profiles of vulnerability and resilience to trauma in brain and blood.Biol Sex Differ. 2020 Mar 30;11(1):13. doi: 10.1186/s13293-020-00288-6. Biol Sex Differ. 2020. PMID: 32228684 Free PMC article.
References
-
- Arimatsu Y, Hatanaka H. Estrogen treatment enhances survival of cultured fetal rat amygdala neurons in a defined medium. Brain Res. 1986;391:151–159. - PubMed
-
- Banasr M, Hery M, Brezun JM, Daszuta A. Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. Eur J Neurosci. 2001;14:1417–1424. - PubMed
-
- Bedard A, Levesque M, Bernier PJ, Parent A. The rostral migratory stream in adult squirrel monkeys: contribution of new neurons to the olfactory tubercle and involvement of the antiapoptotic protein Bcl-2. Eur J Neurosci. 2002;16:1917–1924. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

