Identification, molecular cloning and functional characterization of an octaprenyl pyrophosphate synthase in intra-erythrocytic stages of Plasmodium falciparum
- PMID: 15984931
- PMCID: PMC1317670
- DOI: 10.1042/BJ20050441
Identification, molecular cloning and functional characterization of an octaprenyl pyrophosphate synthase in intra-erythrocytic stages of Plasmodium falciparum
Abstract
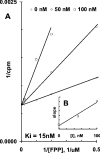
Isoprenoids play important roles in all living organisms as components of structural cholesterol, steroid hormones in mammals, carotenoids in plants, and ubiquinones. Significant differences occur in the length of the isoprenic side chains of ubiquinone between different organisms, suggesting that different enzymes are involved in the synthesis of these side chains. Whereas in Plasmodium falciparum the isoprenic side chains of ubiquinone contain 7-9 isoprenic units, 10-unit side chains are found in humans. In a search for the P. falciparum enzyme responsible for the biosynthesis of isoprenic side chains attached to the benzoquinone ring of ubiquinones, we cloned and expressed a putative polyprenyl synthase. Polyclonal antibodies raised against the corresponding recombinant protein confirmed the presence of the native protein in trophozoite and schizont stages of P. falciparum. The recombinant protein, as well as P. falciparum extracts, showed an octaprenyl pyrophosphate synthase activity, with the formation of a polyisoprenoid with eight isoprenic units, as detected by reverse-phase HPLC and reverse-phase TLC, and confirmed by electrospray ionization and tandem MS analysis. The recombinant and native versions of the enzyme had similar Michaelis constants with the substrates isopentenyl pyrophosphate and farnesyl pyrophosphate. The recombinant enzyme could be competitively inhibited in the presence of the terpene nerolidol. This is the first report that directly demonstrates an octaprenyl pyrophosphate synthase activity in parasitic protozoa. Given the rather low similarity of the P. falciparum enzyme to its human counterpart, decaprenyl pyrophosphate synthase, we suggest that the identified enzyme and its recombinant version could be exploited in the screening of novel drugs.
Figures

Similar articles
-
In vitro and in vivo characterization of a novel insect decaprenyl diphosphate synthase: a two-major step catalytic mechanism is proposed.Biochem Biophys Res Commun. 2013 Dec 6;442(1-2):105-11. doi: 10.1016/j.bbrc.2013.11.025. Epub 2013 Nov 15. Biochem Biophys Res Commun. 2013. PMID: 24246678
-
Cloning and characterization of bifunctional enzyme farnesyl diphosphate/geranylgeranyl diphosphate synthase from Plasmodium falciparum.Malar J. 2013 Jun 4;12:184. doi: 10.1186/1475-2875-12-184. Malar J. 2013. PMID: 23734739 Free PMC article.
-
Characterization of the isoprenoid chain of coenzyme Q in Plasmodium falciparum.FEMS Microbiol Lett. 2002 Jan 22;207(1):13-20. doi: 10.1111/j.1574-6968.2002.tb11021.x. FEMS Microbiol Lett. 2002. PMID: 11886744
-
Plasmodium falciparum glycogen synthase kinase-3: molecular model, expression, intracellular localisation and selective inhibitors.Biochim Biophys Acta. 2004 Mar 11;1697(1-2):181-96. doi: 10.1016/j.bbapap.2003.11.023. Biochim Biophys Acta. 2004. PMID: 15023360 Review.
-
Isoprenoid biosynthesis in the erythrocytic stages of Plasmodium falciparum.Mem Inst Oswaldo Cruz. 2011 Aug;106 Suppl 1:134-41. doi: 10.1590/s0074-02762011000900018. Mem Inst Oswaldo Cruz. 2011. PMID: 21881768 Review.
Cited by
-
Nerolidol: A Sesquiterpene Alcohol with Multi-Faceted Pharmacological and Biological Activities.Molecules. 2016 Apr 28;21(5):529. doi: 10.3390/molecules21050529. Molecules. 2016. PMID: 27136520 Free PMC article. Review.
-
In vivo antimalarial activity and mechanisms of action of 4-nerolidylcatechol derivatives.Antimicrob Agents Chemother. 2015;59(6):3271-80. doi: 10.1128/AAC.05012-14. Epub 2015 Mar 23. Antimicrob Agents Chemother. 2015. PMID: 25801563 Free PMC article.
-
Isoprenoid metabolism in apicomplexan parasites.Curr Clin Microbiol Rep. 2014 Dec 1;1(3-4):37-50. doi: 10.1007/s40588-014-0006-7. Curr Clin Microbiol Rep. 2014. PMID: 25893156 Free PMC article.
-
Isoprenoid biosynthesis in Plasmodium falciparum.Eukaryot Cell. 2014 Nov;13(11):1348-59. doi: 10.1128/EC.00160-14. Epub 2014 Sep 12. Eukaryot Cell. 2014. PMID: 25217461 Free PMC article. Review.
-
Metabolomics and malaria biology.Mol Biochem Parasitol. 2011 Feb;175(2):104-11. doi: 10.1016/j.molbiopara.2010.09.008. Epub 2010 Oct 21. Mol Biochem Parasitol. 2011. PMID: 20970461 Free PMC article. Review.
References
-
- Vial H. J. Isoprenoid biosynthesis and drug targeting in the Apicomplexa. Parasitol. Today. 2000;16:140–141. - PubMed
-
- Chakrabarti D., Da Silva T., Barger J., Paquette S., Patel H., Patterson S., Allen C. M. Protein farnesyltransferase and protein prenylation in Plasmodium falciparum. J. Biol. Chem. 2002;277:42066–42073. - PubMed
-
- Borrmann S., Issifou S., Esser G., Adegnika A. A., Ramharter M., Matsiegui P. B., Oyakhirome S., Mawili-Mboumba D. P., Missinou M. A., Kun J. F., et al. Fosmidomycin-clindamycin for the treatment of Plasmodium falciparum malaria. J. Infect. Dis. 2004;190:1534–1540. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

